Abstract
The macrophyte Egeria densa Planchon, 1849 is a freshwater plant native to the subtropical regions of South America. Fast vegetative reproduction and efficient dispersal allow this species to form extensive beds that produce high levels of oxygen in freshwater ecosystems, generating microhabitats that act as refuge and nursery for an array of organisms, increasing sedimentation and light availability. Despite its undisputable ecological role, it is considered invasive and is present on all continents except Antarctica with the first records in Europe occurring at the 20th century. At the international Minho River (NW Iberian Peninsula), the observation of its presence was noted in the 1990s and is now an established population in this ecosystem. This study is the first descriptive-taxonomical assessment of the associated fauna with the exotic macrophyte E. densa, using both morphological and molecular approaches, three decades after its establishment in the international Minho River. Results indicate the presence of a faunal assemblage, composed a total of 20 identified species, including platyhelminths, hydrozoans, bryozoans, molluscs (Gastropoda), annelids (Oligochaeta and Hirudinea), crustaceans (Ostracoda, Copepoda, Branchiopoda and Amphipoda), aquatic mites and insects. Paludicella aff. articulata (Ehrenberg, 1831), Girardia sinensis Chen & Wang, 2015, Lebertia insignis Neuman, 1880 and Ceriodaphnia rigaudi Richard 1894 are recorded for the first time in Portugal.
Similar content being viewed by others
Introduction
The species Egeria densa Planchon, 1849 is a freshwater perennial plant native to the subtropical regions of South America, which forms dense mats that cover extensive areas. Considered invasive is now present on all continents except Antarctica (Yarrow et al. 2009). Development of E. densa beds lowers water velocity causing sedimentation of suspended particles, increasing light availability (Yarrow et al. 2009). Thus this aquatic plant plays an important role on the structure and function of freshwater environments, generating microhabitats used as refuge and nursery for zooplankton, macroinvertebrates and fish (Pelicice and Agostinho 2006; Yarrow et al. 2009). Furthermore E. densa promotes phytoplankton biomass decline (Mazzeo et al. 2003), stimulates an increase in epiphytic macroinvertebrate biomass which subsequently increases carnivorous fish density (Diehl and Kornijów 1998). The first records in Europe occurred at 20th century (Yarrow et al. 2009), and the first observation at the international Minho River (NW Iberian Peninsula) occurred during the 90ʼs (Antunes, pers. comm.). Since then E. densa developed populations along this freshwater system, generating dense mats on otherwise sandy intertidal environments, forming a microhabitat distinct from its native counterparts that cover mainly the subtidal section. A first attempt at a descriptive-taxonomical assessment of the fauna associated with the exotic macrophyte E. densa at the International Minho River, using both morphological and molecular approaches, provided 12 new records for the study area, including the exotic species Girardia sinensis Chen & Wang, 2015, Menetus dilatatus (Gould 1841) and Helobdella europaea (Kutschera 1985).
Methods
Study area
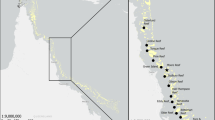
The estuarine area of the international Minho River, located at the northwest Spanish/Portuguese border (NW Iberian Peninsula), has a length of approximately 40 km (Sousa et al. 2008a), with a mesotidal partially mixed system tending towards a salt wedge estuary during the high floods (Sousa et al. 2005). Sampling was performed at the upper section of the estuary, on the Portuguese margin in front of Morraceira Island (Vila Nova de Cerveira), approximately 19 km from the river’s mouth (Fig. 1a). This section of the river is a predominantly freshwater system throughout most of the year, characterized by saline intrusions during the summer season, especially during the dryer months (Pereira et al. 2022) and its margins are covered by monospecific dense mats of Egeria densa (Fig. 1b,c) that overlays the supratidal area, extending to the upper subtidal.
Specimen sampling, identification and preservation
Samplings were performed in the upper section of the international Minho River estuary (Fig. 1), near Morraceira Island, Vila Nova de Cerveira, Portugal. Aerial parts of E. densa were sampled monthly from October 2021 to March 2022, in an intertidal zone, with each sample being collected manually in a area delimited by a square box of 0,1 m2 and bagged for posterior analysis. Species identification was performed using specialized literature (Brinkhurst 1971; Brinkhurst and Jamieson 1971; Lincoln 1979; Cornelius 1995; Alonso 1996; Tachet et al. 2000; Wood 2015; Govedich and Moser 2015; Rogers 2019; Horne et al. 2019; Lee and Lee 2019; Noreña et al. 2019; Conesa-García 2021). All specimens were photographed with a Leica EZ4W stereomicroscope, a Nikon Digital Sight D5-L1 camera using a Nikon SMZ800 stereomicroscope and a Nikon ECLIPSE 50i microscope. Specimens were preserved in 70% ethanol and deposited at the Natural History Museum of the Iberian Peninsula (NatMIP – “Museu de História Natural da Península Ibérica”), sited at Aquamuseu do Rio Minho, Vila Nova de Cerveira, North Portugal.
Genetic analysis
Genomic DNA extraction was performed using a using the E.Z.N.A. Mollusc DNA Kit (Omega Bio-tek), following manufacturer’s instructions. Amplification of the COI-5P region was carried out using the primers LoboF1 (5’–KBTCHACAAAYCAYAARGAYATHGG–3’) and LoboR1 (5’–TAAACYTCWGGRTGWCCRAARAAYCA–3’) (Lobo et al. 2013), and a pre-made PCR mix (VWR International, LLC, Pennsylvania, USA). PCR reactions were comprised of 1× PCR buffer, 1.5 mM of MgCl2, 0.2 mM of the dNTP mixture, 1 U of DNA Taq polymerase, plus 0.5 µM of each primer, ca. 30–50 ng of genomic DNA, and sterile MilliQ-grade water to make up a total volume of 25 µL. The reactions were run on a Hybaid PxE Thermocycler (Thermo Electron Corporation, Milford, Massachusetts), following the PCR conditions in Lobo et al. (2013). PCR products were electrophoresed through a 1% agarose 1× Tris-acetate-EDTA buffer (TAE) gel stained with GreenSafe Premium (Nzytech, Lisbon, Portugal). Positive PCR products were purified using the ExoFast method, in which an enzymatic cleanup that eliminates unincorporated primers and dNTPs is performed with Exonuclease I (Escherichia coli) and FastAP Thermosensitive (SAP), and then sequenced directly. The sequencing reactions were performed using a BigDye Terminator v1.1 from the Applied Biosystems kit (Applied Biosystems, Carlsbad, California), and were run on an ABI3700 DNA analyzer (Perkin-Elmer, Applied Biosystems, Stabvida, Oeiras, Portugal). DNA sequence homology searches and species level identifications were performed using BOLD (Hebert and Ratnasingham 2007) and with BLAST (Altschul et al. 1990) in GenBank databases (Benson 2004). COI sequences were edited and manually aligned using MEGA X (Kumar et al. 2018) and checked for insertions, deletions and stop codons. For phylogenetic reconstruction, COI-5P sequences were retrieved from public databases as follows: sequences for the species in analysis, sequences from the respective genus and sequences from a species of the same family used as outgroup. For the species Bothrioneurum vejdovskyanum, Cypridopsis vidua and Menetus dilatatus, which had no available sequences for congeners, only sequences belonging to members of the same family were retrieved. Specimens of Paludicella aff. articulata and Embolocephalus sp., had no match in BOLD or GenBank. Thus, phylogenetic reconstruction for P. aff. articulata was carried out using sequences belonging the order Ctenostomida and Cyclostomatida for outgroup. For Embolocephalus sp. other sequences from the genus Embolocephalus, as well as from the subfamily Tubificinae were included with Naidinae used as outgroup. Phylogenetic relationships were reconstructed through Maximum-Likelihood, using the substitution model GTR + I + G, as chosen by JModelTest (Posada 2008). MOTUʼs were delimited using the species delineation tool Automatic Barcode Gap Discovery (ABGD) (Puillandre et al. 2012). Intra- and inter-specific distances were calculated on MEGA X using the K2P substitution model.
Results
Morphological analysis
A total of 3370 specimens were examined, representing 20 species belonging to 6 phyla, 16 families and 16 genera. Among these 20 species identified using morphological characters, 4 could only be identified to the family level only (Table 1).
Genetic analysis
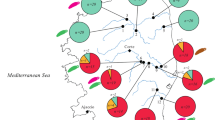
The majority of the taxa examined formed monophyletic MOTUʼs with less than 2% genetic distance namely, Helobdella europaea, Lebertia insignis, Gammarus chevreuxi, Atyaephyra desmarestii, Ischnura elegans, Menetus dilatatus and Girardia sinensis (Figs. 2 and 3). The specimen identified morphologically as Paludicella articulata did not matched any sequence available on BOLD and GenBank, and phylogenetic reconstruction through COI sequences did not reveal any reliable placing or grouping within Ctenostomatida (Fig. 4a). The asexual ostracod Cypridopsis vidua revealed a complex comprised of seven distinct groups (22 MOTUʼs, 7–15% genetic distance), with the specimen analysed nesting within a large cosmopolitan lineage (Group 1), comprised of several distinct MOTUʼs (Fig. 4b). Psectrocladius limbatellus may also be a large cryptic complex with 5 distinct MOTUʼs (14–22% genetic distance) (Fig. 5a). Bothrioneurum vejdovskyanum also formed a possible cryptic complex with three distinctly monophyletic lineages with 4–5% genetic distance, two of which occur sympatrically in the study area (Fig. 5b). Embolocephalus sp. (identified morphologically as Embolocephalus cf. velutinus) is revealed to be a sister taxon of E. velutinus (15% genetic distance) forming a distinct clade within the genus Embolocephalus (Fig. 5c). Exotic species, H. europaea, M. dilatatus and G. sinensis present shared haplotypes spanning multiple continents (Figs. 2a and 3b and c). COI amplification of the hydrozoan Sertularia cupressina failed, revealing a need of specific primers for this group. Physella acuta amplification has shown the presence of chimerical DNA between this gastropod and the epizoic oligochaete Chaetogaster limnaei, which was posteriorly confirmed inside P. acuta mantle cavity.
Maximum-likelihood tree obtained from COI-5P sequences of Psecrtocladius limbatellus species complex (clade highlighted in red) (a); Bothrioneurum vejdovskyanum species complex (different MOTUʼs highlighted by blue, green a red square) (b); Embolocephalus sp. (clade for the genus Embolocephalus highlighted in red and E. veltinus and Embolocephalus sp. in blue) (c). Value at nodes corresponds to bootstrap support (values below 70 are omitted)
Integrated taxonomy (morphology and genetic analysis)
A total of 3370 specimens were examined, representing 20 species belonging to 6 phyla, 16 families and 20 genera. Twelve species constituted new records for the International Minho River (SI). Four species had species level misidentifications, using morphological characters, which could be resolved through the integrative analysis of morphological and genetic features. Another 4 species could only be assertively identified after genetic analysis (Table 1).
Phylum Annelida Lamarck, 1802.
Class Clitellata Michaelsen, 1919.
SubClass Hirudinea Savigny, 1822.
Order Rhynchobdellida Blanchard, 1894.
Family Glossiphoniidae Vaillant, 1890.
Genus Helobdella Blanchard, 1896.
Helobdella europaea (Kutschera 1985) (Fig. 6a).
Type material
Holotype- 1 specimen collected in 1982, deposited at Zoologisches Museum Hamburg (Kutschera 1985).
Type locality
Schobbach/Moosbach, Vörstätten, Germany (Kutschera 1985).
Material examined
28 specimens collected from October to December 2021, on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OP912901.
Geographical distribution
Species with scattered records worldwide (Kutschera 2004).
Distribution in Portugal
International Minho River (this study) and Ferreira River (Valongo, Porto) (Ferreira et al. 2022).
Ecological notes
Freshwater leach, reaching up to 20 mm (Kutschera 1985); predator (Kutschera 1987); sampled in salinities ranging from 0.06 to 0.05, more abundant in October when water temperatures reached 17.75 °C.
Remarks
Kutschera (2004), suggested that this species is exotic to Europe based on how the species COI sequences clustered closely to Helobdella triseralis (Blanchard, 1849) from South America, instead of its European counterparts; haplotypes shared between Europe, Oceania, Asia and North America is indicative of a generalized colonization event originating from a similar founding population (Fig. 3a).
SubClass Oligochaeta Grube, 1850.
Order Tubificida Jamieson, 1978.
Family Naididae Ehrenberg, 1831.
Genus Bothrioneurum Stolč, 1886.
Bothrioneurum vejdovskyanum Štolc 1886 (Fig. 6b).
Type material
Unknown.
Type locality
Štvanice island, Vltava river, Prague, Czech Republic (Štolc 1886).
Material examined
67 specimens, collected October 2021 and from January to March 2022, on E. densa leaves; 2 specimens with COI sequence, GenBank accession number: OP912523 and OP912987.
Geographical distribution
Europe, Asia, North America and Africa (Brinkhurst and Jamieson 1971).
Distribution in Portugal
International Minho River, Ria de Aveiro and Mondego River (Timm and Abarenkov 2021).
Ecological notes
Common on stagnant waters rich in organic matter specially among macrophytes (Dumnicka 2007); sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C.
Remarks
Species level identification based on molecular data; detected the presence of two sympatric lineages with 5% genetic distance in the study area (Fig. 5b).
Genus Embolocephalus Randolph, 1892.
Embolocephalus sp. (Fig. 6c)
Material examined
2 specimens, collected March 2022, on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OP918896.
Ecological notes
Sampled in salinity of 0.04, water temperature 10.74 °C.
Remarks
Genetic analysis shows this specimen as a sister taxon to Embolocephalus velutinus with a 15% distance between the two species, thus forming a distinct clade within the genus Embolocephalus, indicating the need for a revision of this genus in western Europe (Fig. 5c).
Genus Chaetogaster von Baer, 1827.
Chaetogaster cf. diaphanus (Gruithuisen, 1828) (Fig. 6d).
Material examined
1 specimen, collected March 2022, on E. densa leaves.
Geographical distribution
Species recorded in Europe, Africa, North America and Asia (Brinkhurst and Jamieson 1971; Jongwoo 2011).
Distribution in Portugal
Leixões (Azevedo et al. 2020).
Ecological notes
Fresh and brackish water species; maximum size up to 25 mm (Brinkhurst 1971); feeds on algae and zooplankton (Jongwoo 2011); sampled in salinity of 0.04, water temperatures 10.74 °C, inside a tube formed by detritus.
Remarks
This specimen requires further examination to clarify its identification. Nonetheless this is the first record of the genus Chaetogaster for the study area.
Phylum Arthropoda Siebold, 1848.
SubPhylum Chelicerata Heymons, 1901.
Class Arachnida Cuvier, 1812.
Order Trombidiformes Reuter, 1909.
Family Lebertiidae Thor, 1900.
Genus Lebertia Neuman, 1880.
Lebertia insignis Neuman, 1880 (Fig. 7a).
Type material
Holotype missing (Gerecke 2009); Paratypes: Preserved specimens deposited at Forschungsinstitut und Natur-Museum Senckenberg, catalogue numbers 44901-2001-VIE/169, 44902-2005-VIE/169 and 44903-2006-VIE/169 (Senckenberg 2022).
Type locality
Sweden (Gerecke 2009).
Material examined
35 specimens, collected from October 2021 to March 2022, on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OP912882.
Geographic distribution
Central and North Europe with a few records on Southern Europe (Gerecke 2009).
Distribution in Portugal
International Minho River (this study).
Ecological notes
Sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C; found on pools of streams (Sabatino et al. 2010).
Remarks
Species level identification based on molecular data; although the specimen formed a MOTU within the 2% threshold with DNA barcodes of Lebertia insignis from Norway and Montenegro, it forms a clearly distinct lineage (Fig. 2b): the relationship of these lineages may be solved with the analysis of specimens from Southwestern Europe.
SubPhylum Crustacea Brünnich, 1772.
Class Branchiopoda Latreille, 1817.
Order Anomopoda Sars, 1865.
Family Daphniidae Straus, 1820.
Genus Ceriodaphnia Dana, 1853.
Ceriodaphnia rigaudi Richard 1894 (Fig. 7b).
Type material
Unknown.
Type locality
Lao-Kay, Vietnam (Richard 1894).
Material examined
3 specimens, collected October 2021, on E. densa leaves.
Geographical distribution
Commonly distributed in tropical and subtropical regions, more rarely in temperate regions (Alonso 1996).
Distribution in Portugal
International Minho River (this study).
Ecological notes
Maximum body size up to 0.4 mm (Alonso 1996); filter feeder (Alonso 1996); sampled in salinity of 0.06, water temperature 17.75 °C.
Family Eurycercidae Kurz, 1875.
Genus Eurycercus Baird, 1843.
Eurycercus lamellatus (Müller 1776) (Fig. 7c).
Type material
Holotype- Preserved specimen deposited at Natural History Museum of Denmark, catalogue number NHMD-84,865 (Eibye-Jacobsen et al. 2019).
Type locality
Denmark (Müller 1776).
Material examined
6 specimens, collected from January to February 2022, on E. densa leaves.
Geographical distribution
Palaearctic (Alonso 1996).
Distribution in Portugal
International Minho River (this study) and Coimbra (Frey 1971), however details for this second record are lacking.
Ecological notes
Maximum body size up to 3.3 mm (Alonso 1996); filter feeder (Alonso 1996); sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 9.62 to 9.45 °C.
Class Hexanauplia Oakley, Wolfe, Lindgren & Zaharof, 2013.
SubClass Copepoda Milne Edwards, 1840.
Order Cyclopoida Burmeister, 1834.
Family Cyclopidae Rafinesque, 1815.
Genus Macrocyclops Claus, 1893.
Macrocyclops sp. (Fig. 7d)
Material examined
1 specimen collected January 2022, on E. densa leaves.
Ecological notes
Predator (Abdullahi 1992); sampled in salinity of 0.06, water temperature 9.62 °C.
Remarks
Single specimen kept for further morphological analysis. Three species of this genus are reported for Europe, Macrocyclops albidus (Jurine, 1820), Macrocyclops distinctus Richard, 1887 and Macrocyclops fuscus (Jurine, 1820).
Class Malacostraca Latreille, 1802.
Order Amphipoda Latreille, 1816.
Family Gammaridae Leach, 1814.
Genus Gammarus Fabricius, 1775.
Gammarus chevreuxi Sexton, 1913 (Fig. 8a).
Gammarus chevreuxi Sexton, 1913 (a); Atyaephyra desmaresti (Millet, 1831) (b); Procambarus clarkii (Girard, 1852) (c); Cypridopsis vidua (Müller 1776) (d). Scale bars: a-c: 2 mm; d: 0.5 mm
Material examined
77 specimens, collected from October 2021 to March 2022, on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OP913220.
Ecological notes
Sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C.
Remarks
For synonymy details, geographical distribution and distribution in Portugal see Gomes et al. (2022a).
Order Decapoda Latreille, 1802.
Family Atyidae De Haan, 1849.
Genus Atyaephyra de Brito Capello, 1866.
Atyaephyra desmarestii (Millet, 1831) (Fig. 8b).
Material examined
24 specimens, size range 19 to 36 mm, collected from October 2021 to February 2022, on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OP913236.
Ecological notes
Sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C.
Remarks
For synonymy details, geographical distribution and distribution in Portugal see Gomes et al. (2022b).
Family Cambaridae Hobbs, 1942.
Genus Procambarus Ortmann, 1905.
Procambarus clarkii (Girard, 1852) (Fig. 8c).
Material examined
2 juvenile specimens, size range 10 to 13 mm, October 2021, on E. densa leaves.
Ecological notes
Sampled in salinity of 0.06, water temperature 17.75 °C.
Remarks
Common species for the study area; possibly introduced between the end of 80´s and the beginning of the 90´s (Sousa et al. 2013), replacing the native crayfish Austropotamobius pallipes (Sousa et al. 2008b). For synonymy details, geographical distribution and distribution in Portugal see Gomes et al. (2022b).
Class Ostracoda Latreille, 1802.
Order Podocopida Sars, 1866.
Family Cyprididae Baird, 1845.
Genus Cypridopsis Brady, 1867.
Cypridopsis vidua (Müller 1776) (Fig. 8d).
Type material
Holotype- Preserved specimen deposited at Natural History Museum of Denmark, catalogue number NHMD-86,667 (Eibye-Jacobsen et al. 2019).
Type locality
Denmark (Müller 1776).
Material examined
28 specimens collected from October 2021 to January 2022, on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OP912986.
Geographical distribution
Holarctic (Hunt et al. 2007).
Distribution in Portugal
International Minho River (this study); common species in Portuguese freshwater environments (Fernandes Martins et al. 2010).
Ecological notes
Maximum body size up to 500 μm (this study); epifaunal (Horne et al. 2019); nektobenthic, usually on macrophytes (Hunt et al. 2007); deposit feeder (Smith et al. 2015); sampled in salinities ranging from 0.06 to 0.05, water temperatures ranging from 17.75 to 7.69 °C.
Remarks
Species level identification based on molecular data; obtained DNA sequence formed a singleton MOTU, nested within group 1 of Cypridopsis vidua complex (Fig. 4b), comprised of several MOTUʼs. This unusual complex is further described in Cywinska and Hebert (2002).
Subphylum Hexapoda Latreille, 1825.
Order Diptera Linnaeus 1758.
Family Chironomidae Newman, 1834.
Genus Psectrocladius Kieffer, 1906.
Psectrocladius limbatellus (Holmgren 1869) (Fig. 9a).
Type material
Lectotype- Preserved adult specimen deposited at Swedish Museum of Natural History, catalogue number NHRS-GULI000066396 (Holston 2022).
Type locality
Svalbard, Norway (Holmgren 1869).
Material examined
660 specimens (larvae) collected from October 2021 to March 2022, on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OP913217.
Geographical distribution
Holarctic (Stur and Ekrem 2020).
Distribution in Portugal
International Minho River (this study), species recorded between Bragança and Chaves, in River Divor, on Arraiolos (Cobo et al. 2001) and at the Azores archipelago (Murray et al. 2004).
Ecological notes
Sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C.
Remarks
Species level identification based on molecular data; genetic analysis revealed the presence of 5 different MOTUʼs; the sequence obtained nested within a MOTU comprised of exclusively of European sequences (Fig. 5a); minimum and maximum K2P distances between lineages are 14 and 22% respectively.
Order Odonata Fabricius, 1793.
Family Coenagrionidae Kirby, 1890.
Genus Ischnura Charpentier, 1840.
Ischnura elegans (Vander Linden 1820) (Fig. 9b).
Type material
Unknown.
Type locality
Bologna, Italy (Vander Linden 1820).
Material examined
85 specimens (larvae), collected from October 2021 to February 2022, on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OP913230.
Geographical distribution
Europe and Asia, from Iberian Peninsula to Japan (Boudot and Salamun 2015).
Distribution in Portugal
Species recorded in the rivers Minho (this study), Mondêgo (Silva-Santos et al. 2004).
Ecological notes (larvae)
Maximum body size up to 21 mm (Conesa-García 2021); predator (Thompson 1978); sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C.
Remarks
Low genetic distance between Ischnura elegans, Ischnura graelsii and Ischnura saharensis further discussed in Sánchez-Guillén et al. (2014).
Phylum Bryozoa Ehrenberg, 1831.
Order Ctenostomatida Busk, 1852.
Family Paludicellidae Allman, 1885.
Genus Paludicella Gervais, 1836.
Paludicella aff. articulata (Ehrenberg, 1831) (Fig. 9c).
Type material
Unknown.
Type locality
Berlin, Germany (Allman 1856).
Material examined
20 colonies, collected from September 2021 to February 2022, on E. densa leaves; 1 specimen with COI sequence, GenBank accession number OP913190.
Geographical distribution
Cosmopolitan (Rogick and Schalie 1950; Massard and Geimer 2008).
Distribution in Portugal
International Minho River (this study).
Ecological notes
Maximum body size up to 8 cm (Smith et al. 2005); sessile (Loppens 1906; Rogick and Schalie 1950); filter feeder (Riisgård et al. 2010); sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C; colonies on E. densa leaf surface, mostly on senescent individuals.
Remarks
Phylogenetic reconstruction through COI sequences did not reveal any reliable placing or grouping within Ctenostomatida (Fig. 4a).
Phylum Cnidaria Hatschek, 1888.
Class Hydrozoa Owen, 1843.
Order Leptothecata Cornelius, 1992.
Family Sertulariidae Lamouroux, 1812.
Genus Sertularia Linnaeus 1758.
Sertularia cupressina Linnaeus 1758 (Fig. 9d).
Type material
Specimen deposited at the Linnaean Collections, from The Linnean Society of London, catalogue number LINN 1298.5, 1298.6 (Cornelius 1979).
Type locality
“Oceano” (Linnaeus 1758).
Material examined
1 fragmented specimen, collected November 2021, on E. densa leaves.
Geographical distribution
North Atlantic, Baltic Sea to Portugal (MarLIN 2006 BIOTIC; Cornelius 1995).
Distribution in Portugal
International Minho River (this study).
Ecological notes
Maximum body size up to 50 cm; sessile; predator (MarLIN 2006 BIOTIC); Sampled in salinity of 0.06, water temperatures 7.69 °C.
Remarks
Considering that this species is usually found in brackish habitats, its presence at the upper section of the estuary (freshwater) may be explained by upstream transportation during high flood tides.
Phylum Mollusca Linnaeus 1758.
Class Gastropoda Cuvier, 1795.
Family Planorbidae Rafinesque, 1815.
Genus Ancylus Müller, 1773.
Ancylus fluviatilis Müller 1774 (Fig. 10a).
Type material
Syntype- Preserved specimen deposited at Natural History Museum of Denmark, catalogue number NHMD-90,989 (Eibye-Jacobsen et al. 2019).
Type locality
Ilm river, Germany (Müller 1774).
Material examined
4 specimens, size 1.5 mm, collected November 2021 and February 2022 on E. densa leaves.
Geographical distribution
Western Palaearctic (Pfenninger et al. 2003).
Distribution in Portugal
Species recorded in the rivers Minho (Sousa et al. 2005, 2007), Febros (Pinto et al. 2010), Mondêgo (Silva-Santos et al. 2004) and its tributaries (Calapez et al. 2014), Guadiana (Pérez-Quintero 2007) and on Madeira island (Hughes 1995).
Ecological notes
Maximum body size up to 12 mm (Rolán and Otero-Schmitt 1996); grazer (Calow 1973); sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 9.45 to 7.69 °C.
Remarks
Pfenninger et al. (2003) suggested the existence of four cryptic species of Ancylus fluviatilis, three of which occur in Portugal (one at the north region, one at the south and another occurring along the coastal line). (Holyoak et al. 2019) further suggested the revision of the three species described by (Morelet 1845), Ancylus vitraceus Morelet 1845, Ancylus strictus Morelet 1845 and Ancylus obtusus Morelet 1845 as base to formally solve these cryptic lineages.
Genus Menetus Adams & Adams, 1855.
Menetus dilatatus (Gould 1841) (Fig. 10b).
Type material
Syntype- Preserved specimens deposited at Smithsonian Institution, National Museum of Natural History, catalogue number USNM 121,002 (Orrell 2022).
Type locality
USA, Massachusetts, Nantucket island (Gould 1841).
Material examined
1499 specimens collected from October 2021 to March 2022 on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OP913162.
Geographical distribution
Native to North America and introduced in Europe (Czyż et al. 2016).
Distribution in Portugal
Species recorded in the rivers Minho (this study), Mondego and at Praia de Mira (Holyoak et al. 2019).
Ecological notes
Sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C.
Remarks
Shared haplotypes between Europe, Asia and North and Central America is suggests a generalized colonization event originating from a same founding population (Fig. 3b).
Family Physidae Fitzinger, 1833.
Genus Physella Haldeman, 1842.
Physella acuta (Draparnaud 1805) (Fig. 10c).
Type material
Unknown.
Type locality
Garonne River, France (Draparnaud 1805).
Material examined
326 specimens collected from October 2021 to March 2022 on E. densa leaves.
Geographical distribution
Cosmopolitan, native to North America (Lydeard et al. 2016).
Distribution in Portugal
Species recorded in the rivers Minho (Sousa et al. 2005, 2007), Febros (Pinto et al. 2010), Mondêgo (Silva-Santos et al. 2004), Guadiana (Pérez-Quintero 2007), and on Vila Nova de Milfontes (Tornero et al. 2014) (on temporary ponds), at Tavira (França 1921) (unspecified collection data), and on Madeira island (Hughes 1995).
Ecological notes
Maximum body size up to 13 mm (Spyra et al. 2019); grazer (Bernot et al. 2005); sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C; specimens with epizoic oligochaete Chaetogaster limnaei inside the mantle cavity.
Remarks
As this species first description is based on specimens collected in France (Draparnaud 1805), its introduction in Europe probably occurred before the 19th century, with the first record from the Iberian Peninsula dating from 1845 (García-Berthou et al. 2007) and from 1872 at Macaronesian archipelagos (Taylor 2003).
Phylum Platyhelminthes Minot, 1876.
Order Tricladida Lang, 1884.
Family Dugesiidae Ball, 1974.
Genus Girardia Ball, 1974.
Girardia sinensis Chen & Wang, 2015 (Fig. 10d).
Type material
Specimens deposited at Institute of Zoology, Chinese Academy of Sciences, Beijing, China (Chen et al. 2015).
Type locality
Xinghu Lake, Zhaoqing, Guangdong Province, China (Chen et al. 2015).
Material examined
521 specimens collected from October 2021 to March 2022 on E. densa leaves; 1 specimen with COI sequence, GenBank accession number: OQ185384.
Geographical distribution
Scattered records worldwide.
Distribution in Portugal
International Minho River (this study).
Ecological notes
Maximum body size up to 15.2 mm (Chen et al. 2015); sampled in salinities ranging from 0.06 to 0.04, water temperatures ranging from 17.75 to 7.69 °C.
Remarks
Species level identification based on molecular data; shared haplotypes between Europe, Oceania, Asia, North Africa, North and Central America suggests a generalized colonization event originating from small founding populations (Fig. 3c). Considering that the genus Girardia is known for its distribution from North to South America only (Sluys et al. 2005), and based on the phylogenetic placement as a sister taxa to the North American Girardia tigrina and Girardia dorotocephala, G. sinensis is most likely of North American ancestry.
Discussion
Of the 20 species examined, 6 were previously recorded at the Minho River estuary, namely Gammarus chevreuxi (Sousa et al. 2008a), Atyaephyra desmaresti, Procambarus clarkii (Gomes et al. 2022b), Bothrioneurum veydovskyanum (Timm and Abarenkov 2021), Physella acuta and Ancylus fluviatilis (Sousa et al. 2005, 2007). The remaining 12 are reported here for the first time from this geographic location, representing new records of Helobdella europaea, Chaetogaster cf. diaphanus, Embolocephalus sp., Paludicella aff. articulata, Girardia sinensis, Menetus dilatatus, Eurycercus lamellatus, Cypridopsis vidua, Ceriodaphnia rigaudi, Lebertia insignis, Psectrocladius limbatellus and Ischnura elegans. Our record of P. aff. articulata, G. sinensis, L. insignis and C. rigaudi further constitute the first known occurrence of these species in Portuguese territory. Crustacea was the most represented group, accounting for 7 species, while Gastropoda was the most abundant group with 1829 individuals from 3 species examined. A single colony fragment of the hydrozoan species Sertularella cupressina was found on E. densa leaves, however, given that this species is mostly distributed on brackish waters, its record in this study may be the result of upstream transport during strong high flood tides. Rare species, such as (A) fluviatilis, C. rigaudi, E. lamellatus and Macrocyclops sp. may have been underestimated due to their smaller size, as the methodology used was designed to collect larger macroinvertebrates. Exotic species established in the study area include P. clarkii and P. acuta, (previously recorded at the Minho River estuary), and the novel records of M. dilatatus, G. sinensis and H. europaea. These last three species reveal shared haplotypes among different continents (Figs. 2a and 3b and c), possibly resulting from globalized colonization events from similar founding populations (Bodt et al. 2020). Species level identification of Oligochaeta remains rather challenging for non-specialists (Table 1), even with highly detailed taxonomic guides, all specimens collected required further morphological and genetic analysis. The specimens identified as E. cf. velutinus have shown an incongruent identification with known COI-5P sequences for this species (15% genetic distance) which indicates the need for a revision of this genus in western Europe (Fig. 5c). Phylogenetic analysis of (B) veydovskyanum revealed a possible species complex forming three marked monophyletic clades with 4–5% genetic distance, with two of those lineages occurring sympatrically in the study area (Fig. 5b). In turn the sequence of P. aff. articulata, did not retrieve significant percentage of similarity to any other sequence available on BOLD and GenBank. A placement within the order Ctenostomatida was attempted, however COI phylogenetic signal did not produce significant results, with low bootstrap support (Fig. 4a) and erratic topologies, which can be explained by an incipient state of Ctenostomida DNA reference libraries.
Species identification is becoming a highly dynamic process with the addition of DNA barcoding methodologies, forcing the re-evaluation of identifications based on morphological characters in a constant feedback loop. The absence of dichotomous keys for recently introduced exotic species (such as G. sinensis, M. dilatatus and H. europaea) in identification guides clearly hampered our study, as did the lack of historic data regarding macroinvertebrate assemblages before and after the introduction of E. densa in the study area. Furthermore, without the performed genetic analysis, misidentifications (M. dilatatus identified as Gyraulus parvus, G. sinensis identified as Schmidtea sp. and H. europaea identified as Helobdella stagnalis) and incomplete identifications (Table 1), could had further propagate the error on to other efforts developed in the study area. This highlights the necessity of well-prepared DNA reference libraries that can provide points of comparison between species identifications, reduce errors of interpretation and incomplete information provided by most identification guides. Comparing our faunal composition, associated with E. densa, with results obtained by Pedroza-Ramos et al. (2016) on Lake Tota, Colombia, reveals a similar composition at the family level and overlapping genera such as Helobdella, Eurycercus, Cypridopsis, Macrocyclops, Ischnura, Girardia and Physella. Since most studies related to E. densa associated fauna have been performed in freshwater environments (Collier et al. 1999; Pedroza-Ramos et al. 2016), this study is one of the first attempts to characterize taxonomically its interactions on estuarine habitats, and so it is interesting that we found such similarities, especially regarding the American ancestry of some of this genera.
Conclusions
This study represents the first taxonomic characterization of the fauna associated with the macrophyte Egeria densa at the upper section of the international Minho River. Twenty species were recorded, 12 of which constituted new occurrences in the study area, and 4 were exotic species, with the first records for Portugal of the species Paludicella aff. articulata (Ehrenberg, 1831), Girardia sinensis Chen & Wang, 2015, Lebertia insignis Neuman, 1880 and Ceriodaphnia rigaudi Richard 1894. Local fauna catalogues remain largely unfinished, or outdated, and rarely account for the dynamic shifts of macroinvertebrate assemblages related to established bioengineer exotic species, such as E. densa. However, it is important to know this associated biodiversity for a better cause-effect assessment of species introductions and understand its impact on local ecosystems in order to optimise environmental management.
References
Abdullahi BA (1992) Effects of diet on growth and development of three species of cyclopoid copepods. Hydrobiologia 232:233–241. https://doi.org/10.1007/BF00013708
Allman GJ (1856) A monograph of the fresh-water polyzoa: including all the known species, both british and foreign. Ray Society, London
Alonso M (1996) Crustacea, Branchiopoda. In: M.A. R (ed) Fauna Ibérica, vol 7. Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas (CSIC), Madrid, p 486
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Azevedo J, Antunes JT, Machado AM et al (2020) Monitoring of biofouling communities in a portuguese port using a combined morphological and metabarcoding approach. Sci Rep 10:13461. https://doi.org/10.1038/s41598-020-70307-4
Benson DA (2004) GenBank. Nucleic Acids Res 33:D34–D38. https://doi.org/10.1093/nar/gki063
Bernot RJ, Kennedy EE, Lamberti GA (2005) Effects of ionic liquids on the survival, movement, and feeding behaviour of the freshwater snail, Physa acuta. Environ Toxicol Chem 24:1759. https://doi.org/10.1897/04-614R.1
Bodt LH, Rollins LA, Zichello JM (2020) Contrasting mitochondrial diversity of european starlings (Sturnus vulgaris) across three invasive continental distributions. Ecol Evol 10:10186–10195. https://doi.org/10.1002/ece3.6679
Boudot JP, Salamun A (2015) Atlas of the european Dragonflies and Damselflies. KNNV Uitgeverij
Brinkhurst RO (1971) A guide for the identification of british aquatic Oligochaeta, 2nd Editio edn. Freshwater Biological Association, Cumbria
Brinkhurst RO, Jamieson BGM (1971) Aquatic oligochaeta of the world. Oliver & Boyd, Edinburgh
Calapez AR, Elias CL, Almeida SFP, Feio MJ (2014) Extreme drought effects and recovery patterns in the benthic communities of temperate streams. Limnetica 33:281–296
Calow P (1973) The food of Ancylus fluviatilis (Müll.), a littoral stone-dwelling, herbivore. Oecologia 13:113–133. https://doi.org/10.1007/BF00345644
Chen XM, Chen YH, Wu CC, Wang AT (2015) A new species of the genus Girardia (Tricladida: Dugesiidae) from China. Zool Syst 40:166–178. https://doi.org/10.11865/zs.20150202
Cobo F, Soriano O, González MA (2001) Inventario de los Quironómidos (Diptera, Chironomidae) de Portugal. Nov Acta Científica Compostel 11:225–248
Collier KJ, Champion PD, Croker GF (1999) Patch- and reach-scale dynamics of a macrophyte-invertebrate system in a New Zealand lowland stream. Hydrobiologia 392:89–97
Conesa-García MA (2021) Larvas de libélulas en la Península Ibérica. Torres Editores, Granada
Cornelius PFS (1979) A revision of the species of Sertulariidae (Coelenterata: Hydroida) recorded from Britain and nearby seas. Bull Br Museum (Nartural Hist Zool 34:243–321
Cornelius PFS (1995) North-west european thecate hydroids and their Medusae: part 2 Sertulariidae to Campanulariidae: keys and notes for identification of the species. Synopses of the british Fauna. Linnean Society of London, Shrewsbury
Cywinska A, Hebert PDN (2002) Origins of clonal diversity in the hypervariable asexual ostracode Cypridopsis vidua. J Evol Biol 15:134–145. https://doi.org/10.1046/j.1420-9101.2002.00362.x
Czyż MJ, Woliński P, Talarska P, Gołdyn B (2016) Materials to the knowledge of molluscs of Wielkopolska (West-Central Poland). V. Family: Planorbidae (Gastropoda: Pulmonata). Folia Malacol 24:251–264. https://doi.org/10.12657/folmal.024.021
Diehl S, Kornijów R (1998) Influence of submerged macrophytes on trophic interactions among fish and macroinvertebrates. In: Jeppesen S, Søndergaard C (eds) The structuring role of submerged Macrophytes in Lakes. Springer, pp 24–46
Draparnaud JPR (1805) Histoire naturelle des mollusques terrestres et fluviatiles de la France. Levrault & Schoell, Paris
Dumnicka E (2007) Distribution of Oligochaeta in various littoral habitats in the anthropogenic reservoirs. Oceanol Hydrobiol Stud 36:13–19
Ehrenberg CG (1831) Symbolae Physicae, seu Icones et descriptiones Corporum Naturalium novorum aut minus cognitorum, quae ex itineribus per Libyam, Aegyptum, Nubiam, Dongalam, Syriam, Arabiam et Habessiniam ... studio annis 1820-25 redirerunt. Pars Zoologica. v.4. Animalis Evertebrata exclusis Insectis. Berlin
Eibye-Jacobsen D, Pavesi L, Schiøtte T et al (2019) Invertebrates excl. Entomology at the Natural History Museum of Denmark. Version 1.2. Natural History Museum of Denmark. Occurrence dataset. In: Occur. dataset https//doi.org/10.15468/wodhis. https://www.gbif.org/occurrence/2012904487. Accessed 9 Aug 2022
Fernandes Martins MJ, Namiotko T, Cabral MC et al (2010) Contribution to the knowledge of the freshwater Ostracoda fauna in continental Portugal, with an updated checklist of recent and quaternary species. J Limnol 69:160. https://doi.org/10.4081/jlimnol.2010.160
Ferreira S, Oliveira D, da Silva LP et al (2022) First record of the introduced freshwater leech Helobdella europaea Kutschera 1987 in Portugal. Arx Miscel·là nia Zoològica 111–116. https://doi.org/10.32800/amz.2022.20.0111
França C (1921) A preliminary note on bilharziosis indigenous in Portugal. Trans R Soc Trop Med Hyg 15:180–181. https://doi.org/10.1016/S0035-9203(21)90356-0
Frey DG (1971) Worldwide distribution and ecology of Eurycercus and Saycia. Limnol Oceanogr 16:254–308. https://doi.org/10.4319/lo.1971.16.2.0254
García-Berthou E, Boix D, Clavero M (2007) Non-indigenous animal species naturalized in Iberian inland waters. Biological invaders in inland waters: profiles, distribution, and threats. Springer Netherlands, Dordrecht, pp 123–140
Gerecke R (2009) Revisional studies on the european species of the water mite genus Lebertia NEUMAN, 1880 (Acari: Hydrachnidia: Lebertiidae). Abhandlungen der Senckenb Gesellschaft fur Naturforsch 566:1–144
Gomes N, Costa DA, Cantallo H, Antunes C (2022a) Inventario de Amphipoda (Peracarida: Senticaudata y Amphilochidea) del Río Miño Internacional, Península Ibérica. Graellsia 78:e175. https://doi.org/10.3989/graellsia.2022.v78.343
Gomes N, Costa DA, Cantallo H, Antunes C (2022b) Crustaceans (Malacostraca and Thecostraca) from the International Minho River, Iberian Peninsula. Hydrobiology 1:47–75. https://doi.org/10.3390/hydrobiology1010005
Gould AA (1841) Report on the invertebrata of Massachusetts: comprising the mollusca, crustacea, annelida, and radiata. Folsom, Wells and Thurston, Cambridge
Govedich FR, Moser WE (2015) Clitellata: Hirudinida and Acanthobdellida. In: Thorp JH, D. Rogers C (eds) Thorp and Covich’s Freshwater Invertebrates, 4th editio. Academic Press, pp 565–588
Gruithuisen FVP (1828) Über die Nais diaphana und Nais diastropha mit dem Nerven-und Blutsystem derselben. Nova Acta Phys-Med Acad Caes Leop-Carol Nat Cur 14(6):407–420+Plate I
Hebert PDN, Ratnasingham S (2007) The Barcode of Life Data System BOLD. Mol Ecol Notes 7:355–364. https://doi.org/10.1111/j.1471-8286.2006.01678.x
Holmgren AE (1869) Bidrag till kannedomen om Beeren Eilands och Spetsbergens insekt-fauna. K Sven vetenskapsakademiens Handl 8:3–55
Holston K (2022) Entomological collections (NHRS), Swedish Museum of Natural History (NRM). Version 26.782. Swed Museum Nat Hist Occur dataset. https://doi.org/10.15468/fpzyjx
Holyoak D, Holyoak GA, Mendes RMC (2019) A revised check-list of the land and freshwater Mollusca (Gastropoda and Bivalvia) of mainland Portugal. Iberus Rev la Soc Española Malacol 37:113–168
Horne DJ, Meisch C, Martens K (2019) Arthropoda: Ostracoda. Thorp and Covich’s Freshwater Invertebrates. Elsevier, pp 725–760
Hughes SJ (1995) Notes on freshwater mollusca of Madeira including records of species new to the region. Bol do Mus Munic do Funchal 47:23–37
Hunt G, Park LE, Labarbera M (2007) A Novel Crustacean Swimming Stroke: coordinated four-paddled locomotion in the Cypridoidean Ostracode Cypridopsis vidua (Müller). Biol Bull 212:67–73. https://doi.org/10.2307/25066581
Jongwoo J (2011) Naidid oligochaetes (Annelida: Clitellata) from the Seokhyeoncheon and Changreungcheon Streams with New Record of Nais variabilis. Korean J Ecol Environ 44:407–410
Kumar S, Stecher G, Li M et al (2018) MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Kutschera U (1985) Beschreibung einer neuen Egelart, Helobdella striata nov. sp. (Hirudinea: Glossiphoniidae). - Zool Jahrbücher Syst 112:469–476
Kutschera U (1987) Notes on the taxonomy and biology of leeches of the genus Helobdella Blanchard 1896 (Hirudinea: Glossiphoniidae). Zool Anz 219:321–323
Kutschera U (2004) The freshwater leech Helobdella europaea (Hirudinea: Glossiphoniidae): an invasive species from. South America? Lauterbornia 52:153–162
Lee DJ, Lee W (2019) Arthropoda: Copepoda. Thorp and Covich’s Freshwater Invertebrates. Elsevier, pp 761–780
Lincoln RJ (1979) Brithish marine Amphipoda: Gammaridea. British Museum (Natural History), London
Linnaeus C (1758) Systema naturae per regna tria naturae:secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, Editio dec. Impensis Direct. Laurentii Salvii, Holmiæ [Stockholm]
Lobo J, Costa PM, Teixeira MAL et al (2013) Enhanced primers for amplification of DNA barcodes from a broad range of marine metazoans. BMC Ecol 13:34. https://doi.org/10.1186/1472-6785-13-34
Loppens K (1906) Bryozoaires marins et fluviatiles de la Belgique. Ann la Société R Zool Malacol Belgique 41:286–321
Lydeard C, Campbell D, Golz M (2016) Physa acuta Draparnaud, 1805 should be treated as a native of North America, not Europe. Malacologia 59:347–350. https://doi.org/10.4002/040.059.0213
MarLIN 2006 BIOTIC Biological Traits Information Catalogue. Marine Life Information Network. Plymouth: Marine Biological Association of the United Kingdom
Massard JA, Geimer G (2008) Global diversity of bryozoans (Bryozoa or Ectoprocta) in freshwater: an update. Bull la Société des Nat Luxemb 109:139–148
Mazzeo N, Rodríguez-Gallego L, Kruk C et al (2003) Effects of Egeria densa Planch. Beds on a shallow lake without piscivorous fish. Hydrobiologia 506–509:591–602. https://doi.org/10.1023/B:HYDR.0000008571.40893.77
Morelet A (1845) Description des mollusques terrestres et fluviatiles du Portugal. Baillière, Paris
Müller OF (1774) Vermivm terrestrium et fluviatilium, seu animalium infusoriorum, helminthicorum et testaceorum, non marinorum, succincta historia. apud Heineck et Faber, Havniæ
Müller OF (1776) Zoologiae Danicae prodromus: seu Animalium Daniae et norvegiae indigenarum; characteres, nomina, et synonyma imprimis popularium / auctore Othone Friderico Müller … Impensis auctoris. typis Hallageriis, Havniae
Murray DA, Hughes SJ, Furse MT, Murray WA (2004) New records of Chironomidae (Diptera: Insecta) from the Azores, Macaronesia. Ann Limnol - Int J Limnol 40:33–42. https://doi.org/10.1051/limn/2004004
Noreña C, Porfiriev A, Timoshkin O (2019) Phylum Platyhelminthes. Thorp and Covich’s Freshwater Invertebrates. Elsevier, pp 113–144
Orrell T (2022) NMNH Extant Specimen Records (USNM, US). Version 1.58. In: Natl. Museum Nat. Hist. Smithson. Institution. Occur. dataset https//doi.org/10.15468/hnhrg3. https://www.gbif.org/dataset/821cc27a-e3bb-4bc5-ac34-89ada245069d. Accessed 9 Aug 2022
Pedroza-Ramos A, Caraballo P, Aranguren-Riaño N (2016) Estructura trófica de los invertebrados acuáticos asociados a Egeria densa (Planch. 1849) en el lago de tota (Boyacá-Colombia). Intropica 11:21. https://doi.org/10.21676/23897864.1858
Pelicice FM, Agostinho AA (2006) Feeding ecology of fishes associated with Egeria spp. patches in a tropical reservoir, Brazil. Ecol Freshw Fish 15:10–19. https://doi.org/10.1111/j.1600-0633.2005.00121.x
Pereira H, Sousa MC, Vieira LR et al (2022) Modelling Salt intrusion and estuarine plumes under climate change scenarios in two transitional ecosystems from the NW Atlantic Coast. J Mar Sci Eng 10:262. https://doi.org/10.3390/jmse10020262
Pérez-Quintero JC (2007) Diversity, habitat use and conservation of freshwater molluscs in the lower Guadiana River basin (SW Iberian Peninsula). Aquat Conserv Mar Freshw Ecosyst 17:485–501. https://doi.org/10.1002/aqc.796
Pfenninger M, Staubach S, Albrecht C et al (2003) Ecological and morphological differentiation among cryptic evolutionary lineages in freshwater limpets of the nominal form-group Ancylus fluviatilis (O.F. Müller, 1774). Mol Ecol 12:2731–2745. https://doi.org/10.1046/j.1365-294X.2003.01943.x
Pinto AL, Varandas S, Coimbra AM et al (2010) Mullet and gudgeon liver histopathology and macroinvertebrate indexes and metrics upstream and downstream from a wastewater treatment plant (Febros River-Portugal). Environ Monit Assess 169:569–585. https://doi.org/10.1007/s10661-009-1197-x
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256. https://doi.org/10.1093/molbev/msn083
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol 21:1864–1877
Richard J (1894) Sur quelques animaux inferienres des eaux douces du Tonkin (Protozoaires, Rotiferes, Entomostraces). Mem la Soc Zool Fr 7:237–243
Riisgård HU, Okamura B, Funch P (2010) Particle capture in ciliary filter-feeding gymnolaemate and phylactolaemate bryozoans - a comparative study. Acta Zool 91:416–425. https://doi.org/10.1111/j.1463-6395.2009.00417.x
Rogers DC (2019) Phylum Mollusca. In: Thorp and Covich’s Freshwater Invertebrates, 4th editio. Elsevier, pp 309–355
Rogick MD, Schalie HV (1950) Studies on fresh-water Byrozoa. XVII, Michigan Bryozoa. Ohio J Sci 50:136–146
Rolán E, Otero-Schmitt J (1996) Guía dos Moluscos de Galicia. Galaxia, Vigo
Sabatino A, Di, Gerecke R, Gledhill T, Smit H (2010) Süßwasserfauna von Mitteleuropa, Bd. 7/2–2 Chelicerata. Springer Berlin Heidelberg, Berlin, Heidelberg
Sánchez-Guillén RA, Córdoba-Aguilar A, Cordero-Rivera A, Wellenreuther M (2014) Genetic divergence predicts reproductive isolation in damselflies. J Evol Biol 27:76–87. https://doi.org/10.1111/jeb.12274
Senckenberg (2022) Collection Arachnology SMF. In: Occur. dataset https://doi.org/10.15468/fiz2j5. https://www.gbif.org/dataset/96596036-f762-11e1-a439-00145eb45e9a. Accessed 9 Aug 2022
Silva-Santos P, Oliveira SV, Cortes RM, Albuquerque A (2004) Natural and anthropogenic variations in a channelized water course in Centre of Portugal. Limnetica 23:257–270
Sluys R, Kawakatsu M, Ponce de León R (2005) Morphological stasis in an old and widespread group of species: contribution to the taxonomy and biogeography of the genus Girardia (Platyhelminthes, Tricladida, Paludicola). Stud Neotrop Fauna Environ 40:155–180. https://doi.org/10.1080/01650520500070220
Smith AM, Brunton MA, Batson PB (2005) Infestation of a temperate reservoir by freshwater bryozoans: an integrated research programme. In: Moyano H, Cancino J, Jackson PW (eds) Bryozoan Studies 2004 Proceedings of the 13th International Bryozoology Association conference, Concepcion/Chile, 11–16 January 2004. CRC Press, London, p 10
Smith AJ, Horne DJ, Martens K, Schön I (2015) Class Ostracoda. Thorp and Covich’s Freshwater Invertebrates. Elsevier, pp 757–780
Sousa R, Guilhermino L, Antunes C (2005) Molluscan fauna in the freshwater tidal area of the River Minho estuary, NW of Iberian Peninsula. Ann Limnol - Int J Limnol 41:141–147. https://doi.org/10.1051/limn/2005009
Sousa R, Antunes C, Guilhermino L (2007) Species composition and monthly variation of the Molluscan fauna in the freshwater subtidal area of the River Minho estuary. Estuar Coast Shelf Sci 75:90–100. https://doi.org/10.1016/j.ecss.2007.02.020
Sousa R, Dias S, Freitas V, Antunes C (2008a) Subtidal macrozoobenthic assemblages along the River Minho estuarine gradient (north-west Iberian Peninsula). Aquat Conserv Mar Freshw Ecosyst 18:1063–1077. https://doi.org/10.1002/aqc.871
Sousa R, Dias S, Guilhermino L, Antunes C (2008b) Minho River tidal freshwater wetlands: threats to faunal biodiversity. Aquat Biol 3:237–250. https://doi.org/10.3354/ab00077
Sousa R, Freitas FEP, Mota M et al (2013) Invasive dynamics of the crayfish Procambarus clarkii (Girard, 1852) in the international section of the River Minho (NW of the Iberian Peninsula). Aquat Conserv Mar Freshw Ecosyst 23:656–666. https://doi.org/10.1002/aqc.2323
Spyra A, Cieplok A, Strzelec M, Babczyńska A (2019) Freshwater alien species Physella acuta (Draparnaud, 1805) - a possible model for bioaccumulation of heavy metals. Ecotoxicol Environ Saf 185:109703. https://doi.org/10.1016/j.ecoenv.2019.109703
Štolc A (1886) Přehled českých Tubificidů. Sitzungsberichte der königlichen Böhmischen Gesellschaft für Wissenschaften. Praha 1885:640–647
Stur E, Ekrem T (2020) The Chironomidae (Diptera) of Svalbard and Jan Mayen. Insects 11:183. https://doi.org/10.3390/insects11030183
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2000) Invertébrés d’Eau Douce. Systématique, Biologie, Écologie. CNRS Editions, Paris
Taylor DW (2003) Introduction to Physidae (Gastropoda: Hygrophila); biogeography, classification, morphology. Rev Biol Trop 51:1–287
Thompson DJ (1978) The natural prey of larvae of the damselfly, Ischnura elegans (Odonata: Zygoptera). Freshw Biol 8:377–384. https://doi.org/10.1111/j.1365-2427.1978.tb01458.x
Timm T, Abarenkov K (2021) World distribution of the aquatic Oligochaeta. In: PlutoF. Occur. dataset https//doi.org/10.15468/2ywn3u. https://www.gbif.org/dataset/e1539f14-f749-4f73-8c18-3355277f94f4. Accessed 9 Aug 2022
Tornero I, Sala J, Gascón S et al (2014) Aquatic macrofauna of Vila Nova de Milfontes temporary ponds, with the first record of Cyphon hilaris Nyholm, 1944 (Coleoptera: Scirtidae) from Portugal. Boletín la Soc Entomológica Aragon 55:326–330
Vander Linden PL (1820) Agriones Bononienses descriptae. Typographia annesii de nobilibus, Bononiae
Wood TS (2015) Phyla Ectoprocta and Entoprocta (Bryozoans). In: Thorp JH, Rogers DC (eds) Thorp and Covich’s Freshwater Invertebrates, 4th editio. Academic Press, pp 327–345
Yarrow M, Marín VH, Finlayson M et al (2009) The ecology of Egeria densa Planchon (Liliopsida: Alismatales): a wetland ecosystem engineer. Rev Chil Hist Nat 82:299–313. https://doi.org/10.4067/S0716-078X2009000200010
Acknowledgements
This research was funded by national funds through the Foundation for Science and Technology (FCT), within the scope of the project PTDC/BIA-BMA/6363/2020.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
Conceptualization: Nuno Gomes; Methodology: Nuno Gomes, Dimítri de Araújo Costa, Carlos Antunes; Formal analysis and investigation: Nuno Gomes, Dimítri de Araújo Costa, Duarte Martins, Sónia Rocha; Writing - original draft preparation: Nuno Gomes; Writing - review and editing: Dimítri de Araújo Costa, Duarte Martins, Sónia Rocha, Isabel Sousa Pinto, Carlos Antunes; Resources: Carlos Antunes; Supervision: Isabel Sousa Pinto, Carlos Antunes.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gomes, N., de Araújo Costa, D., Martins, D. et al. Insights into the fauna associated with Egeria densa at the upper section of the international Minho River estuary (NW Iberian Peninsula) 3 decades after its establishment. J Coast Conserv 27, 36 (2023). https://doi.org/10.1007/s11852-023-00962-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11852-023-00962-y