Abstract
While it is common knowledge that Red Drum (Sciaenops ocellatus) inhabit oligohaline waters (salinity <5), lifetime reconstructions of salinity histories have been lacking, and this study provides unique insight into interannual and ontogenetic patterns of oligohaline occupancy by this economically valuable sportfish. Growth consequences of oligohaline exposure and the relationship of oligohaline residency with river discharge were also investigated. Oligohaline exposure varied most during years 2 and 3 of life. During this time, 22% (n = 26/120 individuals) of Red Drum were oligohaline residents (≥ 90% of these years spent in oligohaline salinities), 34% (n = 41) were meso-polyhaline residents (< 10% of years 2 and 3 spent in oligohaline waters), and 44% (n = 53) spent time in both oligohaline and meso-polyhaline salinities. Trends in oligohaline residency match putative Red Drum life history. Oligohaline residents were present during years 1–6 of life; however, oligohaline residency peaked during the second year of life (n = 37, 31%) and by year 7 no oligohaline residents remained. Growth of oligohaline resident Red Drum during years 2–3 of life was lower than non-resident fish. However, long-term growth consequences of oligohaline residency were not apparent. The proportion of oligohaline residents during years 2 or 3 of life was positively related to river discharge. This divergence in salinity residency by juvenile Red Drum demonstrates that life history diversity was present in this population and that oligohaline waters provided important nursery habitat for fish that successfully recruited to the adult population.
Similar content being viewed by others
Introduction
Life history diversity in the habitats occupied within animal populations can have important consequences for species distribution, abundance, and resilience to changing environmental conditions (Hilborn et al. 2003; Kerr et al. 2010; Schindler et al. 2010). Traditionally, intrapopulation diversity in habitat use has been observed through the occurrence of partial migrations and identification of resident and migratory groups within a population, originally in avian taxa (Mayr 1926; Berthold 1999; Chapman et al. 2011) and more recently in fishes (Jonsson and Jonsson 1993; Chapman et al. 2012a; Chapman et al. 2012b). Estuarine dependent fishes are typically euryhaline occupying a wide range of salinities (Able 2005). This trait may result in life history diversity in salinities that fishes inhabit throughout life and during distinct life periods, like the juvenile phase (Able 2005; Kerr et al. 2009; Secor and Kerr 2009). In fishes, this diversity has been described by the population contingent hypothesis where different contingents exhibit varying migratory strategies across or residency within salinity thresholds (Clark 1968; Secor 1999; Nims and Walther 2014). In contrast, fish populations may also contain individuals demonstrating continuous gradients of salinity variation without identifiable contingent structure, exhibiting facultative use of variable salinities throughout life (Cucherousset et al. 2005; Rohtla and Vetemaa 2016). Importantly, either discrete or continuous differences in estuarine habitat use and salinity exposure may be equally important for the resilience of populations to ongoing environmental change.
Salinity in estuaries is inherently variable, can be affected by both climatic and anthropogenic drivers, and salinity fluctuations are an osmoregulatory challenge for fishes. Seasonal changes in estuarine salinity ranges and distribution are common in estuaries like Mobile Bay, AL that receive high, but variable river discharge throughout the year (Dzwonkowski et al. 2011; Coogan and Dzwonkowski 2018; Coogan et al. 2020). Climate change has the potential to augment these seasonal patterns through increased extreme rainfall events, frequency, and intensity of storms (Scavia et al. 2002; Gillanders et al. 2011). Sea level rise will change estuaries through increased marine water input and potential loss of marsh habitat through submergence of land (Scavia et al. 2002; Gillanders et al. 2011; Anderson et al. 2014). Estuarine salinity ranges are also under anthropogenic alteration, freshwater inflow is augmented through upstream water demand and flood control (Pringle et al. 2000; Kimmerer 2002) and dredging activities can increase saltwater intrusion (Schroeder and Wiseman Jr. 1999). Salinity fluctuations are a physiological challenge for fishes, because osmoregulatory machinery has to be switched to handle conditions that fluctuate from below to above isosmotic equilibrium (Morgan et al. 1997; McDonald and Grosell 2006; Watson et al. 2014). Continual metabolic costs are incurred when fishes inhabit salinities far from isosmotic equilibrium (Boeuf and Payan 2001) and even euryhaline fishes have decreased growth in fresh and oligohaline waters (Lankford and Targett 1994; Secor et al. 2000; Sampaio and Bianchini 2002). Maintaining adequate growth is an important component of fish early life history, given that elevated growth rates contribute to higher survival and successful recruitment to adult populations (Houde 1987; Houde 1997; Sogard 1997). To ensure population persistence in a changing environment, knowledge of population diversity in salinity exposure, salinity tolerance, and osmoregulatory fitness consequences are needed.
One way to infer past salinity exposure of fishes is to use otolith elemental ratios as salinity proxies. Otoliths grow continuously with fish growth and deposit calcium carbonate (CaCO3) in an annular ring structure that can be used to age fishes (Campana and Neilson 1985; Campana 1999). During CaCO3 accretion, other elements potentially indicative of the surrounding environment can be incorporated and remain in the otolith after deposition (Campana 1999; Sturrock et al. 2012; Walther and Limburg 2012). For elemental ratios to be used as salinity proxies the dissolved fraction in ambient waters must vary predictably with salinity changes and incorporate into otoliths proportionally to environmental availability (Elsdon et al. 2008). The ratio of strontium (Sr) to calcium (Ca) is a common salinity proxy given that dissolved Sr:Ca typically increases asymptotically with salinity (Phillis et al. 2011; Walther and Limburg 2012); however, this is not always the case (Kraus and Secor 2004; Brown and Severin 2009). In Mobile Bay Alabama, both Sr and Ca have positive conservative relationships with salinity and the Sr:Ca ratio increases asymptotically with salinity (Nelson and Powers 2020a). The Sr:Ca partition coefficient from water to juvenile Red Drum (Sciaenops ocellatus, Linnaeus) otoliths is consistent across salinity in the lab (Nelson and Powers 2019) and the field (Nelson and Powers 2020a) and otolith Sr:Ca varies predictably with salinity across a wide temperature range (Nelson and Powers 2020a). Although past work has found Red Drum otolith Sr:Ca to be unrelated to salinity (Rooker et al. 2004), our validation studies (Nelson and Powers 2019, 2020a) demonstrate that Sr:Ca can identify oligohaline salinity exposure within coastal Alabama and other estuaries with similar dissolved ambient ratio trends.
Red Drum (Sciaenops ocellatus, Linnaeus) are an estuarine dependent euryhaline fish that utilize a wide salinity range across juvenile and adult life stages. Adult Red Drum spawn in the fall near passes (inlets) that connect estuaries to marine waters (Holt et al. 1983; Overstreet 1983; Holt et al. 1989). Typically, spawning occurs in nearshore marine waters (Holt et al. 1989; Lowerre-Barbieri et al. 2019), but spawning aggregations have been observed in high salinity estuarine waters (Lowerre-Barbieri et al. 2008; Reyier et al. 2011). After spawning tidal currents carry eggs and larvae into estuaries (Holt et al. 1983; Holt et al. 1989), where larval Red Drum (≥ 4 mm Standard Length [SL]) settle (15–20 days post hatch) across seagrass, saltmarsh, and nonvegetated bottoms (Holt et al. 1983; Rooker and Holt 1997; Stunz et al. 2002). Although post-settlement Red Drum are typically encountered in salinities greater than 20 (Rooker and Holt 1997; Stunz et al. 2002), by 9 mm SL low salinity exposure (< 1) is tolerated (Crocker et al. 1981), and juveniles are found throughout estuaries from fresh to marine waters (Bacheler et al. 2008; Bacheler et al. 2009a; Dance and Rooker 2016). While not as common, juvenile Red Drum are also encountered in nearshore marine habitats (Winner et al. 2014; Hightower et al. 2016). This wide-ranging juvenile salinity exposure is possible given that Red Drum can almost instantaneously regulate internal ion concentrations in response to abrupt salinity shifts, even between fresh and marine waters (Watson et al. 2014). After maturation, which occurs from ages 3–6 (Wilson and Nieland 1994), adult Red Drum typically migrate down the estuary to high salinity inshore, nearshore, and offshore waters (Overstreet 1983; Winner et al. 2014; Lowerre-Barbieri et al. 2019). However, adult Red Drum have been collected throughout Mobile Bay in salinities ≤5 (Powers et al. 2012; Hightower et al. 2016; Livernois et al. 2020). Therefore, Red Drum may exhibit diversity in salinity exposure and habitats occupied during both juvenile and adult stages.
Red Drum populations drastically decreased throughout the Gulf of Mexico during the 1980s given increased commercial harvest to meet demand for the popular blackened redfish dish (Powers et al. 2012). To rebuild populations, Red Drum harvest was banned in federal waters in 1988 (Gulf of Mexico Fishery Management Council 1988; Porch 2000; Hightower et al. 2016). Since commercial harvest cessation, a valuable recreational fishery has developed that primarily targets juvenile fish (1–4 years old) in inshore waters (Porch 2000; Nelson and Powers 2020b). This fishery is tightly regulated to ensure that 30% of juveniles escape the inshore fishery and contribute to the adult spawning stock (Powers and Burns 2010; Powers et al. 2012). Although Red Drum inhabit a wide salinity range, there is limited knowledge on how oligohaline salinity exposure varies throughout the population during both juvenile and adult life stages. Furthermore, fitness consequences of oligohaline exposure and drivers of oligohaline residency are also unknown. To ensure persistence of this popular sportfish, this knowledge is needed and will help elucidate Red Drum essential fish habitat, the importance of oligohaline waters to adult Red Drum production, and how fish may respond to fluctuating salinities within estuaries. Therefore, the objectives of this study were to: 1) quantify age specific oligohaline salinity exposure of Red Drum, 2) determine if Red Drum growth was related to oligohaline salinity exposure, and 3) determine if oligohaline residency was related to river discharge into the estuary.
Methods
Fish collection and otolith processing
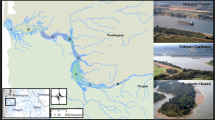
Adult Red Drum were collected from large schools in nearshore Alabama waters during fall 2014 via purse seine as part of a concurrent age and growth study (Hightower et al. 2016). Collection sites were directly adjacent to Mobile Bay/Mississippi Sound Alabama (Fig. 1). A spotter pilot located Red Drum schools and a fishing vessel deployed a purse seine (0.55 km long, 39.62 m high, 2.54 cm mesh) enclosing each school. Once the net was pursed, a random sample of Red Drum were removed, passed to a small research vessel, and transported back to the Dauphin Island Sea Lab (DISL). Three schools were sampled during this period; 129 fish were collected from the first school on October 28th, 128 fish were collected from a second school on October 29th, and 211 fish were collected from the third school on October 31st (Fig. 1).
Collection locations of adult Red Drum schools and the depth averaged salinity of coastal Alabama waters predicted with the Environmental Fluid Dynamics Code (Kim and Park 2012) from June 2008–2010
The left sagittae from each Red Drum was used to age fish and a subset was selected for otolith chemical analysis. Otoliths were sectioned on a Hillquist thin-sectioning petrographic saw, mounted to glass slides, and polished using 0.3 μm alumina powder to obtain a smooth surface for otolith aging and chemical analysis. All fish were aged by two independent readers and fish age was determined by counting the number of opaque annuli and assigning an integer age. Red Drum deposit annuli in the winter and have easily discernable rings (VanderKooy 2009), which consistently leads to high agreement among readers (Powers et al. 2012; Hightower et al. 2016). After aging, an attempt was made to randomly select 20 males and 20 females from each school for otolith chemical analysis. To be included in the selection process, fish had to be at least 10 years old, which slightly skewed the sex ratios sampled from each school (n = 120, Table 1). The aging average percent error (Beamish and Fournier 1981) from these 120 otoliths was 0.67%, equaling >99% agreement among readers. For any otolith where the two readers did not agree (n = 15), both readers reviewed the otolith together and reached a consensus on the final age. Prior to chemical analysis, multiple otoliths were adhered to a single slide, triple rinsed with ultrapure (18.2 MΩ-cm) water, scrubbed for one minute with an acid cleaned toothbrush, and triple rinsed with ultrapure water once more. Otoliths were dried and stored in a laminar flow clean hood until chemical analysis.
Otolith chemical and increment analysis
Elemental concentrations (43Ca and 88Sr) of otoliths were quantified with laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) at the DISL instrumentation lab with a 213 nm Nd:YAG NWR laser coupled to a Agilent 7700x quadrupole ICPMS. Lifetime otolith profiles were obtained with straight line laser ablation tracks. These profiles originated in the core, continued along the sulcus, and passed through each annulus until the otolith edge was reached. For each profile a pre-ablation pass was performed with 20% energy, 5 Hz repetition rate, 40 μm spot size, and a speed of 100 μm/s. Each ablation used 30% energy, 10 Hz repetition rate, a spot size of 25 μm, and moved at a speed of 5 μm/s, resulting in an approximate output energy of 5 J/cm2. Duplicate runs of certified reference materials (CRM) NIST-612 and MACS-3 were performed before analysis began and after each subsequent hour of analysis. The NIST-612 was used to asses analytical precision (RSD 88Sr = 8.3%) and sixty seconds of background signal were obtained before ablations of both CRMs and otoliths. Limits of detection calculation, background signal removal, instrument drift correction, and conversion of raw elemental counts to concentrations (ppm) were performed in Iolite version 3 (Igor version 6.37) using the Trace Element IS data reduction scheme with Ca as the internal standard (37.69%) and MACS-3 as the CRM (Longerich et al. 1996; Paton et al. 2011). The ppm concentrations of Sr were converted to molar ratios with Ca prior to statistical analysis.
Otoliths were imaged and ablation transects were measured with Olympus cellSens software to determine the year of life that ablation transect data corresponded to. Straight-line laser ablation transects were measured in μm from their origin (otolith core) to the middle of each annuli and the otolith edge. Continuous ablation elemental data was converted from time (output every 0.512 s) to distance (μm) by multiplying the output timestamp by 5 μm/s (the speed of the laser). If ablation transect data fell from the otolith core (ablation origin) to the middle of the first annulus it received a 1, representing the first year of life. If data occurred from the middle of the first annulus to the middle of the second it received a 2, and this continued at each subsequent annuli until the otolith edge was reached. Calendar year was assigned to profile increments by back-dating from the time of capture (fall 2014) and it was assumed that annuli deposition occurred in the winter (VanderKooy 2009).
Given that annuli measurements were along straight-line ablation tracks, these measurements could be used to predict fish length-at-age with otolith back calculation. Predicted length at a given age was obtained using the Dahl-Lea method
where Li is the back-calculated length of a fish at age i, Lc is the length of fish at capture, Oi is the length of the otolith from the ablation origin (otolith core) to the ith annulus, and Oc is the length from the otolith core to the otolith edge along the laser track (Quist et al. 2012). The proportional nature of this method accounted for potential bias among otolith accretion measurements resulting from section and transect variability. Therefore, predicted fish length and predicted fish growth within a given year of life were used for all growth analyses.
Data processing, oligohaline thresholds, and inference statistics
Prior to all analyses, outliers were removed and data was smoothed using a loess regression. Outliers in each Ele:Ca profile were identified as data points greater than four times the 3rd quartile of the entire ablation. This was a conservative approach to remove outliers, but clearly filtered out erroneous data that was likely a result of instrument noise rather than ecologically relevant values (Smith et al. 2013; McMillan et al. 2017). After outlier removal all data was passed through a local polynomial regression (loess), using the loess package in R with a span equal to 0.025. This low span smoothed high frequency variation that are too fine to be used for environmental interpretation, a common approach in LA-ICPMS analysis (Sinclair et al. 1998; Lowe et al. 2011; Nims and Walther 2014), but retained ecologically-relevant Sr:Ca variations. All analyses and oligohaline exposure inference were performed using the smoothed data from the loess regression.
Past Red Drum oligohaline salinity (< 5) exposure was inferred using otolith Sr:Ca as a salinity proxy. Otolith Sr:Ca partition coefficients (DSr mean = 0.22 [0.213–0.227 95% CI]) were multiplied by their respective water Sr:Ca ratios (mean = 7.5 mmol:mol [7.31–7.71 95% CI]) at a salinity of 5 (Morse and Bender 1990; Nelson and Powers 2019, 2020a), to generate low (1.56 mmol:mol), mean (1.65 mmol:mol), and high (1.75 mmol:mol) oligohaline thresholds. Multiple thresholds were used to ensure that overall exposure patterns were robust to threshold variation. Within a given year of life, the number of ablation transect points below oligohaline thresholds was divided by the total number of transect points within that year to infer the percentage of time fish spent in oligohaline waters at each threshold value. Combined percentages were also calculated for years 1–4 and 2–3, given initial patterns observed in the data. Fish that spent ≥90% of a year of life in oligohaline waters were considered oligohaline residents during that year and fish that spent <10% of a year in oligohaline waters were considered meso-polyhaline residents. The same criteria were used to classify fish throughout years 1–4 and 2–3 of life. For the purposes of this study, oligohaline waters were defined as those with salinity <5 and meso-polyhaline waters were those ≥5 regardless of where these waters occurred.
Growth analyses
To determine if Sr:Ca was related to age-specific juvenile Red Drum growth, linear regressions were performed for years 1–4 of life, with annual predicted fish growth as the dependent variable and mean annual Sr:Ca as the independent variable. Predicted fish growth within a given year of life was obtained by subtracting the predicted length of fish at age i-1 from the length of fish at age i. For the first year of life, growth was the same as back calculated fish length. A similar approach was taken to determine if the proportion of time spent in oligohaline waters (as inferred from Sr:Ca thresholds) was related to fish growth during years 1–4 of life. These linear models were run for each threshold percentage classifications to determine if results were robust to the threshold chosen.
After initial data review, it was apparent that a large proportion of Red Drum were oligohaline residents during years 2 and 3 of life (≥ 90% of time in oligohaline waters). To determine if combined growth differed among oligohaline residents and all others in the study during these years, a t-test with each threshold value (low, mean, high) was run. The combined predicted fish growth during years 2–3 of life was the sum of growth from each year. To determine if oligohaline residency early in life affected lifetime growth trajectories, differences in oligohaline resident (during yrs. 2–3) growth and all other fish were further investigated with von Bertalanffy growth models,
where Lt is the predicted length at a given age (t), L∞ is the asymptotic maximum length reached by individuals in the population, K is a growth coefficient that adjusts how quickly the asymptotic length is reached, and t0 is an extrapolation to fix the curve to the x-axis. These models were run with each threshold classification to determine if results were sensitive to variable thresholds. To ensure that Red Drum sex specific growth (Powers et al. 2012) did not bias comparisons among salinity groupings, growth among sexes or salinity groupings were allowed to vary within models. The most parsimonious model comparing sex or salinity groupings was selected as the one with the lowest corrected Akaike information criterion (AICc), unless a simpler model was within 2 AICc units. Bootstrapped 95% confidence intervals were obtained using the nlsBoot function (5000 iterations) in R (Baty et al. 2015) for each parameter estimate from the best fitting models. Differences in collection age distributions between oligohaline resident Red Drum (during yrs. 2–3) and all other fish were tested with the Kolmogorov-Smirnov (K-S) test, and Chi-squared (X2) tests were used to determine if oligohaline residency assignment was independent of sex and collection school.
Analysis of oligohaline residency with river discharge
The relationship of oligohaline residency during years 2 and 3 of life and river discharge was investigated with generalized linear models (GLM) in R using the Gamma family and log link. The freshwater discharge into Mobile Bay was obtained by adding the daily discharge at Coffeeville and Claiborne lock and dams (waterdata.usgs.gov) and generating annual means for 1990–2008. This mean annual discharge was the independent variable in GLMs. The response variable was the proportion of oligohaline resident Red Drum during years 2 or 3 of life in each calendar year. For each calendar year, this proportion was the number of oligohaline residents during years 2 or 3 of life, divided by the total number of Red Drum present during years 2 or 3 of life. These proportions only included individuals from otolith chemical analysis and at least ten fish, whose second or third year of life corresponded to a respective calendar year, needed to be present for inclusion of that year. Therefore, only years 1993 through 2006 were used for analysis and GLMs were run with oligohaline classifications based on each threshold value.
Results
Lifetime Sr:Ca profiles and salinity inference
All Red Drum had otolith Sr:Ca ratios that increased as fish aged (Fig. 2, Online Resources 1, 2, 3). The largest increases occurred during years 4–6 of life and after this Sr:Ca increase, the mean adult (years ≥7) Sr:Ca (3.55 mmol:mol ± 0.01 SE) across all individuals was almost double the value predicted at 35 psu using 0.22 DSr (1.83 mmol:mol). Profiles among individuals were similar after this increase and exhibited periodicity throughout the duration of life (Online Resources 1, 2, 3). The majority of juvenile (years 1–4 of life) Sr:Ca ratios were within expected ranges and individual variability in Sr:Ca was present. Sr:Ca below oligohaline thresholds (Fig. 2a, Online Resource 1), near thresholds (Fig. 2b, Online Resource 2), and above thresholds (Fig. 2c, Online Resource 3) was observed among juvenile Red Drum.
Example lifetime otolith Sr:Ca ratios of individual Red Drum that, during years 2–3 of life, were classified as (a) oligohaline residents (≥ 90% of the time in salinities <5); (b) spent substantial time in oligohaline and meso-polyhaline salinities (≥10% and < 90% in oligohaline waters); and (c) meso-polyhaline residents (< 10% in oligohaline waters). The mean oligohaline threshold value was used for classification (horizontal dashed lines) and the gray shaded box represents the range between low and high thresholds. Fish age is on the x-axis of each panel and tick marks correspond with measured annuli locations
The largest differences among Red Drum Sr:Ca and inferred salinity exposure occurred during the second and third years of life. During year 2, 37 (low = 31, high = 43) Red Drum were classified as oligohaline residents and 37 (low = 48, high = 29) were meso-polyhaline residents. The 46 (low = 41, high = 48) remaining Red Drum spent between 10% and < 90% of their time in oligohaline waters (Fig. 3a). During their third year of life, more Red Drum were meso-polyhaline residents (low = 60, mean = 51, high = 39); however, 24 (low = 22, high = 30) were oligohaline residents (Fig. 3a). During years 2–3 of life combined, 26 (low = 23, high = 31) Red Drum were oligohaline residents, 41 (low = 53, high = 31) were meso-polyhaline residents, and 53 (low = 44, high = 58) spent between 10% and < 90% of their time in oligohaline waters (Fig. 3b). Some Red Drum also resided in oligohaline salinities during years 1–6 of life and years 1–4 combined (Fig. 3); however, after year 6 no within-year oligohaline residents were present. While these patterns of oligohaline salinity exposure were present, 100% of fish spent some portion of life in oligohaline waters according to the mean and high thresholds. When the low threshold was used, only 3 fish were classified as never entering oligohaline waters. While the absolute number of age-specific oligohaline residents varied in the above analyses, the overall patterns and trends remained, demonstrating robust results to variable thresholds tested here.
Number of Red Drum grouped by the percentage of time they spent in (a) oligohaline waters (salinity <5) during years 1–6 of life; (b) during combined years 1–4 and 2–3 of life. Lines on each bar correspond to the range of number of individuals classified with low and high oligohaline threshold values
Growth
Annual Red Drum growth during years 1–3 of life was unrelated to otolith Sr:Ca or the percentage of time spent in oligohaline waters. All models for each of these three years had R2 < 0.03, and p values >0.07 indicating that no correlations were present (Fig. 4a - 4c, 4e - 4 g). During the fourth year of life, a negative relationship with growth and otolith Sr:Ca (R2 = 0.18, p < 0.001, Fig. 4d) and a positive relationship with growth and the percentage of time spent in oligohaline waters were present (all threshold R2 < 0.1, all p < 0.001, Fig. 4h). However, R2 were still low in these models, indicating weak relationships. The models regressing growth with the percentage of time in oligohaline waters did have clustering of points at extreme values, which could have biased analyses. However, the residual vs. leverage plots of these models indicated that these points were not overly influential.
Although continuous within-year growth relationships were not detected, combined growth during years 2–3 was lower for oligohaline residents then for others across all threshold values (all t > 2.6, all p < 0.01, Fig. 5a). This slower growth was also apparent in von Bertalanffy models, oligohaline resident Red Drum (during yrs. 2–3 of life) had lower K values across all thresholds (Table 2, Fig. 5b). von Bertlannaffy models among sex indicated that female Red Drum had a higher L∞ (Table 2, Online Resource 4). However, individual assignment to oligohaline resident (during yrs. 2–3) was independent of sex (all X2 > 0.86, all p > 0.12) and collection school (all X2 > 0.75, all p > 0.53), and age distributions did not differ between oligohaline residents or others (all D < 0.15, all p > 0.78). Furthermore, growth results were robust to variable thresholds, given that results did not differ among threshold choice.
The mean ± 1 standard error (SE) of predicted Red Drum growth of oligohaline resident (< 5) and non-resident (≥ 5) fish classified with low, mean, and high threshold values during their second and third year of life (a). The back calculated lengths at each age for oligohaline resident and non-resident Red Drum during years 2–3 of life and the predicted growth curves obtained from the most parsimonious von Bertalanffy growth equation using the mean threshold value (b)
Oligohaline residency and river discharge
The proportion of oligohaline resident Red Drum during years 2 or 3 of life was positively related to discharge from corresponding calendar years, across all oligohaline thresholds (all p ≤ 0.02, Fig. 6). For each m3s−1 increase in discharge, the proportion of oligohaline residents was predicted to increase by 1.001 times (Table 3). Obtaining the same results across thresholds demonstrates that discharge results are robust to the thresholds tested here.
Annual mean discharge (dashed line) for each calendar year and the proportion of Red Drum classified as oligohaline residents during their second or third year of life within each corresponding calendar year (a). Vertical lines correspond to the range of proportions obtained among low, mean, and high oligohaline thresholds. Example Red Drum otolith, laser ablation track, and corresponding calendar years for an age 12 Red Drum (b). The measurement length (mm) from the otolith core to the first annuli is included for scale
Discussion
Red Drum habitat use and salinity exposure has previously been assessed with field surveys and tagging studies that focused on sub-adult fish (Bacheler et al. 2009a; Bacheler et al. 2009b; Dance and Rooker 2016). These studies were temporally restrictive, given that fish had to be captured or detected for insight to be drawn. While it is common knowledge Red Drum may inhabit oligohaline waters (Bacheler et al. 2009a; Bacheler et al. 2009b), lifetime reconstructions of salinity histories have been lacking, and the results in this study provide unique insight into interannual and ontogenetic patterns of oligohaline occupancy by this important species. Salinity exposure varied most during years 2 and 3 of life. In this time period, 22% of Red Drum were oligohaline residents, 34% were meso-polyhaline residents, and 44% spent years 2 and 3 in both oligohaline waters and higher salinities. After age 2, oligohaline occupancy decreased and by age 7, only one Red Drum spent any time in oligohaline waters. This divergence in salinity residency by juvenile Red Drum demonstrates that life history diversity was present in this population and that oligohaline waters provided important nursery habitat for fish that successfully recruited to the adult population.
Considerations and caveats
When using otolith chemical markers as salinity proxies there are many important considerations and caveats that must be addressed (Elsdon et al. 2008). First and foremost is the choice of elemental proxy or proxies that are used. In Mobile Bay AL, the relationship of salinity with ambient Sr and Ca is conservative and does not change with variable discharge into the estuary (Nelson and Powers 2020a). Therefore, the water Sr:Ca salinity relationship is also unaffected by discharge and exhibits temporally stable discernable differences among oligohaline and meso-polyhaline salinities (Nelson and Powers 2020a). In contrast, Ba is not temporally conservative and peaks during periods of low discharge (Nelson and Powers 2020a). Although Ba:Ca was quantified in Red Drum otoliths, this non-conservative behavior was the first reason why Ba:Ca was not used as a salinity proxy in this study. The second was that Ba:Ca partition (DBa) into Red Drum otoliths was not consistent across salinities, while DSr was (Nelson and Powers 2019). Consistent DSr indicates that ambient water chemical shifts are responsible for otolith Sr:Ca changes, which are not confounded by salinity effects beyond changing water chemistry. Although Rooker et al. 2004 found no salinity effect on juvenile Red Drum otolith Sr:Ca, their study did not quantify underlying water Sr:Ca variability among salinity treatments. It is possible that no variation existed, leading to their insignificant results. Discernable differences in water Sr:Ca among salinities and consistent DSr are important assumptions (Elsdon et al. 2008; Walther and Limburg 2012) that were met for Red Drum in coastal Alabama (Nelson and Powers 2019, 2020a).
Another important consideration is the lag time between salinity shifts and otolith chemical signature stabilization. Previous studies have found that it takes 12 to 30 days (Lowe et al. 2009; Macdonald and Crook 2010; Miller 2011) for otolith chemical signatures to stabilize after environmental changes. However, discernable changes in otolith composition after environmental shifts have been observed within 1–3 days (Miller 2011; Hoover et al. 2012). Entire otolith scans of juvenile Red Drum Sr:Ca indicate that otolith composition rapidly changes with salinity shifts (Nelson and Powers 2019). However, even if otolith Sr:Ca instantaneously shifts with changing salinity, enough otolith material must be deposited for a change to be detected with a given laser spot size (Nims and Walther 2014). Like all fishes, as Red Drum age the amount of time incorporated by a fixed laser size increases, because Red Drum growth rate decreases (Powers et al. 2012). Therefore, as fish age more time must be spent in a chemically unique environment for it to be detected in the otolith. This time compression within laser spot size could be why oligohaline exposure was not detected in the adult portion of Red Drum otoliths. Rapid juvenile fish movements among oligohaline and meso-polyhaine waters may have also been undetected because of time lags and the loess smoothing we applied. Although this is the case, our approach provided insight into the dominant age-specific salinity ranges inhabited by Red Drum.
In addition to ambient water chemistry, other exogenous and endogenous factors such as diet, temperature, growth, and ontogeny may affect otolith elemental ratios, potentially confounding the use of Sr:Ca as a salinity proxy (Sturrock et al. 2012; Izzo et al. 2018; Reis-Santos et al. 2018). Although dietary sources can augment Sr:Ca, water has been found to be the dominant source of otolith Sr (Walther and Thorrold 2006; Webb et al. 2012; Doubleday et al. 2013). Furthermore, prey consumed in oligohaline waters likely have Sr concentrations that reflect these waters (Nims and Walther 2014). Even if Red Drum otolith Sr:Ca was slightly elevated in oligohaline salinities through consuming prey with Sr concentrations indicative of higher salinities, this would have decreased our estimates of oligohaline water exposure. Therefore, our estimates of oligohaline exposure are conservative with respect to dietary confounding.
Both positive (Bath et al. 2000; Martin et al. 2004; Nelson et al. 2018) and negative (Townsend et al. 1992; DiMaria et al. 2010; Miller and Hurst 2020) correlations of otolith DSr with temperature have been found. Decoupling the interactive effects of fish growth and temperature is also difficult given that fish growth is linked to temperature and somatic growth rate is negatively related to DSr (Sadovy and Severin 1992; Walther et al. 2010; Miller and Hurst 2020). Consistent DSr for field collected juvenile Red Drum across a wide temperature range (Nelson and Powers 2020a) demonstrates that temperature confounding effects were minimal. Red Drum throughout the estuary should also experience similar seasonal temperature fluctuations. Therefore differences among individuals in oligohaline exposure inferred from Sr:Ca should be unaffected by temperature bias even if it was present. There is the possibility that oligohaline resident Red Drum during years 2–3 had higher DSr then other fish, given that their growth rate was lower within this time period. This would have resulted in higher otolith Sr:Ca during these years, which once again indicates that our estimates of oligohaline exposure and residency are conservative at least in juvenile fish.
One final consideration was our choice to use threshold analysis and the values selected as part of this analysis. Although impacts of confounding variables on otolith Sr:Ca were likely minimal, we cannot conclude they were nonexistent, and as such, cannot assign exact salinities to otolith Sr:Ca. Furthermore, otolith and water Sr:Ca in Mobile Bay does not exhibit changes beyond salinities of 10 (Nelson and Powers 2020a). This asymptotic Sr:Ca relationship is typical among estuaries; however, differences in oligohaline and meso-polyhaline salinity ranges are discernable with this proxy (Walther and Limburg 2012; Seeley and Walther 2018; Nelson and Powers 2020a). The choice of an oligohaline threshold was also physiologically and ecologically relevant. Red Drum residency within oligohaline portions of estuaries has been poorly studied and these salinities are below Red Drum isosmotic equilibrium (Crocker et al. 1983; Forsberg and Neill 1997; Watson et al. 2014). While thresholds cannot determine precise salinities, they provide probable conservative estimates of inhabited salinity ranges, especially when results are robust across variable thresholds (Nims and Walther 2014; Seeley and Walther 2018), as was the case here. While we are confident that juvenile Red Drum otolith Sr:Ca can reliably discern oligohaline waters from meso-polyhailne ones, we do believe that growth and maturation likely affected otolith Sr:Ca ratios in adult Red Drum and discuss these ontogenetic implications in the following section.
Ontogenetic patterns of oligohaline exposure
The percentage of oligohaline resident Red Drum exhibited an inverse pattern to Red Drum maturation. Red Drum mature during years 3–6 of life (Wilson and Nieland 1994) and after maturation move down the estuary to join spawning aggregations (Overstreet 1983; Winner et al. 2014; Lowerre-Barbieri et al. 2019). The percentage of within-year oligohaline residents decreased during their third year of life, and few fish were classified as oligohaline residents past year 4, the time when most fish have matured by (Wilson and Nieland 1994). Therefore, the trend of decreasing oligohaline salinity exposure with age matched expected Red Drum life history.
Although movement to higher salinities was expected after maturation, otolith Sr:Ca may have been affected by maturation and/or decreased somatic growth. Around maturation timing (during yrs. 3–6), otolith Sr:Ca increased approaching values nearly double the maximum predicted from water elemental curves and juvenile partition coefficients (Nelson and Powers 2019, 2020a). As discussed above, an increase in Sr:Ca is expected given known migrations associated with maturation. However, this large increase of Sr:Ca around the age of maturity is likely a result of increased otolith Sr:Ca partition (DSr). Otolith Sr:Ca can increase with respect to gonad development in otoliths, blood, and endolymph (Kalish 1991; Sturrock et al. 2014; Sturrock et al. 2015) and increased Sr:Ca with age has been shown in both resident freshwater and marine fishes (Brown and Severin 2009; Loewen et al. 2016). Furthermore, steady increases of Sr:Ca with fish age have been observed in Red Drum (Hoff and Fuiman 1993; Fuiman and Hoff 1995) and Whitemouth Croaker (Micropogonias furnieri), another estuarine dependent Sciaenid (Albuquerque et al. 2012). The age of maturation is also when Red Drum growth rapidly decreases (Porch 2000; Powers et al. 2012), which as discussed above also increases DSr. Although age-specific trends in oligohaline exposure matched putative Red Drum life history, the confounding effects of growth and maturation on Sr:Ca, coupled with laser time compression (discussed above), could have led to erroneous salinity inference in adult otolith regions.
Future validation work is needed to elucidate the drivers behind increased adult otolith Sr:Ca and observed periodicity within this time period. After this Sr:Ca increase, regular variations with peaks and valleys that could be driven by internal physiological processes or external environmental changes were observed. One avenue for this work would be to investigate whether otolith Sr:Ca co-varies with magnesium calcium ratios (Mg:Ca). Otolith Mg:Ca is a likely indicator of metabolic activity and growth that is unaffected by the surrounding environment (Limburg et al. 2018). If otolith Sr:Ca and Mg:Ca have similar patterns of periodicity than a physiological driver on otolith Sr:Ca is likely. One way to disentangle many confounding effects on Sr:Ca would be to use otolith Sr87:Sr86 ratios as salinity proxies, given that no significant biological fractionation occurs in Sr87:Sr86 incorporation (Kennedy et al. 2000; Walther and Limburg 2012). If adult Red Drum spend enough time in oligohaline waters for it to be detectable with a given laser spot size then otolith Sr87:Sr86 should elucidate this exposure.
Growth and drivers of oligohaline residency
Slower growth among oligohaline resident Red Drum and other fish during years 2–3 of life was clearly shown through mean comparisons and lower von Bertalanffy growth coefficients (K). The metabolic costs of inhabiting salinities below their isosmotic equilibrium (Boeuf and Payan 2001), which is near 10 salinity (Crocker et al. 1983; Forsberg and Neill 1997; Neill et al. 2004), likely led to this slower growth rate; a common trend among euryhaline species (Lankford and Targett 1994; Secor et al. 2000; Sampaio and Bianchini 2002). The largest consequence of slower growth during this life stage is that oligohaline resident Red Drum may remain in the harvestable slot limit for a longer duration, potentially increasing vulnerability to recreational harvest and oligohaline mortality rates (Nelson and Powers 2020b).
While differences in juvenile growth were present, the lack of continuous growth relationships and the shared L∞ in von Bertalanffy models, indicates growth effects were minimal and did not result in long-term growth consequences. The osmoregulatory capability of Red Drum likely contributed to these minimal growth consequences of oligohaline salinity residency. Within 8 h of transfer from sea water to freshwater, Red Drum are able to downregulate sea water excretion pathways and rely on existing ion uptake channels to maintain muscle ion and water balance (Watson et al. 2014). This rapid osmoregulatory response is unique among fishes, 95% of teleosts are stenohaline (McCormick 2001), and some euryhaline species (i.e. Mozambique Tilapia [Oreochromis mossambicus, Peters] and Southern Flounder) take 4 days or longer to adjust to rapid salinity shifts incurring stressful metabolic costs (Morgan et al. 1997; Tipsmark et al. 2008). This osmoregulation capability likely contributed to minimal growth consequences of oligohaline exposure allowing for prolonged residency periods within these waters.
Although back calculation of length from otolith incremental analysis can under estimate fish length and older individuals are usually predicted to be smaller at a given age than younger fish (Ricker 1975; Quist et al. 2012), the equal distribution of collection age among oligohaline resident individuals (during yrs. 2–3) and other fish in should remove any age bias in growth comparisons. Female Red Drum also have larger L∞ than males (Powers et al. 2012), which was detected in our sex-specific von Bertalanffy model. However, Red Drum sex and collection school should not bias salinity growth models given that assignment to oligohaline residency (during yrs. 2–3) was independent of these variables. The L∞ and K estimates from von Bertalanffy models were also similar to those obtained from observed fish length at age data (Powers et al. 2012), indicating that otolith back calculation did not introduce large biases in growth models. Instead, this approach was likely an appropriate way to account for otolith accretion measurement bias, introduced through otolith section and transect variability.
The facultative use of oligohaline habitat by juvenile Red Drum may be a strategy to alleviate negative density dependent interactions. Movement of age 1 Red Drum to upper and lower estuarine habitats was hypothesized to alleviate the negative relationship of juvenile Red Drum growth and abundance (Bacheler et al. 2012). It is possible that similar density dependent mechanisms operate in Mobile Bay. Larval Red Drum typically settle in meso-polyhaline portions of the estuary (Rooker and Holt 1997; Stunz et al. 2002) and initially, these areas may provide plentiful resources. However, as juvenile Red Drum grow and age requiring more food (Neill et al. 2004) some fish may move into potentially less exploited oligohaline waters. This hypothetical mechanism may explain why oligohaline residency was highest during years 2–3 of life. Perhaps the minimal growth consequences of oligohaline residency are less than those that would be present if Red Drum remained in crowded portions of the estuary. This density dependent driver of oligohaline residency in Gulf of Mexico estuaries could be investigated in the future with new field studies or analysis of long-term monitoring data.
Oligohaline residency is likely driven by both physical and ecological processes, because the proportion of oligohaline resident Red Drum during years 2 or 3 of life was positively related to river discharge. The oligohaline proportion of Mobile Bay increases with elevated river discharge into this estuary (Coogan and Dzwonkowski 2018). Therefore, if Red Drum move up the estuary to alleviate negative density dependent effects, then a higher proportion of fish would be exposed to oligohaline waters during high discharge periods. There may also be a genetic component behind oligohaline residency, because Red Drum genetic divergence in the Gulf of Mexico is likely driven by discharge into estuaries (Hollenbeck et al. 2019). Although the exact mechanism behind juvenile Red Drum oligohaline residency remains unknown, future research should strive to elucidate these mechanisms, shedding light on drivers of life history diversity in euryhaline fishes.
Contingent vs. continuum and conclusions
Although life history diversity in oligohaline exposure of juvenile Red Drum exists, this diversity does not fit the population contingent hypothesis as seen in other euryhaline fishes like Striped Bass (Morone saxatilis, Walbaum) (Secor 1999; Zlokovitz et al. 2003; Morissette et al. 2016), White Perch (Morone americana, Gmelin) (Kerr et al. 2009), and Southern Flounder (Paralichthys lethostigma, Girard) (Nims and Walther 2014). For Striped Bass and White Perch, a contingent of individuals within the population remained resident in freshwater throughout life while other individuals migrated to mesohaline or polyhaline salinities (Secor et al. 2001; Kerr et al. 2009). The opposite pattern is present in Southern Flounder, a contingent remained in meso-polyhaline salinities throughout life, while others made single or multiple migrations into oligohaline waters (Nims and Walther 2014). In contrast, Red Drum spent time in both oligohaline and meso-polyhaline salinities, and life history diversity in oligohaline exposure was only present in juvenile fish. This continuous use of estuarine nursery habitats provides resiliency to the inevitable future fluctuations of estuarine salinity (Scavia et al. 2002; Gillanders et al. 2011; Tupitza and Glaspie 2020). Facultative occupancy of oligohaline salinities is likely present in other estuarine dependent fishes and should be investigated further, elucidating resiliency in other economically valuable species.
Change history
24 April 2021
The original online version of this article was revised due to a retrospective Open Access order and to correct the affiliation of the fourth author, Benjamin D. Walther from "Department of Life Sciences, Texas A&M University, 6300 Ocean Drive, Corpus Christi, TX 78412, USA" to "Department of Life Sciences, Texas A&M University - Corpus Christi, 6300 Ocean Drive, Corpus Christi, TX 78412, USA".
04 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10641-021-01096-6
References
Able KW (2005) A re-examination of fish estuarine dependence: evidence for connectivity between estuarine and ocean habitats. Estuar Coast Shelf Sci 64(1):5–17
Albuquerque CQ, Miekeley N, Muelbert JH, Walther BD, Jaureguizar AJ (2012) Estuarine dependency in a marine fish evaluated with otolith chemistry. Mar Biol 159(10):2229–2239
Anderson JB, Wallace DJ, Simms AR, Rodriguez AB, Milliken KT (2014) Variable response of coastal environments of the northwestern Gulf of Mexico to sea-level rise and climate change: implications for future change. Mar Geol 352:348–366
Bacheler NM, Buckel JA, Paramore LM (2012) Density-dependent habitat use and growth of an estuarine fish. Can J Fish Aquat Sci 69(11):1734–1747
Bacheler NM, Paramore LM, Buckel JA, Hightower JE (2009a) Abiotic and biotic factors influence the habitat use of an estuarine fish. Mar Ecol Prog Ser 377:263–277
Bacheler NM, Paramore LM, Buckel JA, Scharf FS (2008) Recruitment of juvenile red drum in North Carolina: spatiotemporal patterns of year-class strength and validation of a seine survey. N Am J Fish Manag 28(4):1086–1098
Bacheler NM, Paramore LM, Burdick SM, Buckel JA, Hightower JE (2009b) Variation in movement patterns of red drum (Sciaenops ocellatus) inferred from conventional tagging and ultrasonic telemetry. Fish Bull 107(4):405–420
Bath GE, Thorrold SR, Jones CM, Campana SE, McLaren JW, Lam JWH (2000) Strontium and barium uptake in aragonitic otoliths of marine fish. Geochim Cosmochim Acta 64(10):1705–1714
Baty F, Ritz C, Charles S, Brutsche M, Flandrois J-P, Delignette-Muller M-L (2015) A toolbox for nonlinear regression in R: the package nlstools. J Stat Softw 66(5)
Beamish R, Fournier D (1981) A method for comparing the precision of a set of age determinations. Can J Fish Aquat Sci 38(8):982–983
Berthold P (1999) A comprehensive theory for the evolution, control and adaptability of avian migration. Ostrich 70(1):1–11
Boeuf G, Payan P (2001) How should salinity influence fish growth? Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 130(4):411–423
Brown RJ, Severin KP (2009) Otolith chemistry analyses indicate that water Sr: Ca is the primary factor influencing otolith Sr: Ca for freshwater and diadromous fish but not for marine fish. Can J Fish Aquat Sci 66(10):1790–1808
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297
Campana SE, Neilson JD (1985) Microstructure of fish otoliths. Can J Fish Aquat Sci 42(5):1014–1032
Chapman BB, Brönmark C, Nilsson J-Å, Hansson L-A (2011) The ecology and evolution of partial migration. Oikos 120(12):1764–1775
Chapman BB, Hulthen K, Brodersen J, Nilsson PA, Skov C, Hansson LA, Bronmark C (2012a) Partial migration in fishes: causes and consequences. J Fish Biol 81(2):456–478
Chapman BB, Skov C, Hulthen K, Brodersen J, Nilsson PA, Hansson LA, Bronmark C (2012b) Partial migration in fishes: definitions, methodologies and taxonomic distribution. J Fish Biol 81(2):479–499
Clark J (1968) Seasonal movements of striped bass contingents of Long Island sound and the New York bight. Trans Am Fish Soc 97(4):320–343
Coogan J, Dzwonkowski B (2018) Observations of wind forcing effects on estuary length and salinity flux in a river-dominated, microtidal estuary, Mobile Bay, Alabama. J Phys Oceanogr 48(8):1787–1802
Coogan J, Dzwonkowski B, Park K, Webb B (2020) Observations of Restratification after a wind mixing event in a shallow highly stratified estuary. Estuar Coasts 43(2):272–285
Crocker PA, Arnold CR, DeBoer JA, Holt GJ (1983) Blood osmolality shift in juvenile red drum, Sciaenops ocellatus L exposed to fresh water. Journal of Fish Biology 23(3):315–319
Crocker PA, Arnold CR, Holt JD, DeBoer JA (1981) Preliminary evaluation of survival and growth of juvenile red drum (Sciaenops ocellata) in fresh and salt water. Journal of the World Mariculture Society 12(1):122–134
Cucherousset J, Ombredane D, Charles K, Marchand F, Baglinière J-L (2005) A continuum of life history tactics in a brown trout (Salmo trutta) population. Can J Fish Aquat Sci 62(7):1600–1610
Dance MA, Rooker JR (2016) Stage-specific variability in habitat associations of juvenile red drum across a latitudinal gradient. Mar Ecol Prog Ser 557:221–235
DiMaria RA, Miller JA, Hurst TP (2010) Temperature and growth effects on otolith elemental chemistry of larval Pacific cod, Gadus macrocephalus. Environ Biol Fish 89(3):453–462
Doubleday ZA, Izzo C, Woodcock SH, Gillanders BM (2013) Relative contribution of water and diet to otolith chemistry in freshwater fish. Aquat Biol 18(3):271–280
Dzwonkowski B, Park K, Kyung Ha H, Graham WM, Hernandez FJ, Powers SP (2011) Hydrographic variability on a coastal shelf directly influenced by estuarine outflow. Cont Shelf Res 31(9):939–950
Elsdon TS, Wells BK, Campana SE, Gillanders BM, Jones CM, Limburg KE, Secor DH, Thorrold SR, Walther BD (2008) Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanogr Mar Biol Annu Rev 46(1):297–330
Forsberg JA, Neill WH (1997) Saline groundwater as an aquaculture medium: physiological studies on the red drum, Sciaenops ocellatus. Environ Biol Fish 49(1):119–128
Fuiman LA, Hoff GR (1995) Natural variation in elemental composition of sagittae from red drum. J Fish Biol 47(6):940–955
Gillanders BM, Elsdon TS, Halliday IA, Jenkins GP, Robins JB, Valesini FJ (2011) Potential effects of climate change on Australian estuaries and fish utilising estuaries: a review. Mar Freshw Res 62(9):1115–1131
Gulf of Mexico Fishery Management Council. 1988. Amendment Number 2 and Environmental Assessment and Regulatory Impact review and Initial Regulatory Flexability Analysis to the Fishery Management Plan For The Red Drum Fishery of the Gulf of Mexico URL: http://gulfcouncil.org/wp-content/uploads/REDDRUM-Amend-02-Final-1988-03.pdf
Hightower CL, Drymon JM, Powers SP (2016) Current status of adult red drum (Sciaenops ocellatus) in the north Central Gulf of Mexico: an update of abundance, age composition, and mortality estimates. SEDAR49-DW-16. SEDAR, North Charleston, SC. 19
Hilborn R, Quinn TP, Schindler DE, Rogers DE (2003) Biocomplexity and fisheries sustainability. Proc Natl Acad Sci 100(11):6564–6568
Hoff GR, Fuiman LA (1993) Morphometry and composition of red drum otoliths: changes associated with temperature, somatic growth rate, and age. Comp Biochem Physiol A Physiol 106(2):209–219
Hollenbeck CM, Portnoy DS, Gold JR (2019) Evolution of population structure in an estuarine-dependent marine fish. Ecol Evol 9(6):3141–3152
Holt SA, Holt GJ, Arnold CR (1989) Tidal stream transport of larval fishes into non-stratified estuaries. Rapp PV Reun Cons Int Explor Mer 191:100–104
Holt SA, Kitting CL, Arnold CR (1983) Distribution of young red drums among Different Sea-grass meadows. Trans Am Fish Soc 112(2B):267–271
Hoover RR, Jones CM, Grosch CE, Gillanders BM (2012) Estuarine ingress timing as revealed by spectral analysis of otolith life history scans. Can J Fish Aquat Sci 69(8):1266–1277
Houde ED (1987) Fish early life history dynamics and recruitment variability. Am Fish Soc Symp 2:17–29
Houde ED (1997) Patterns and trends in larval-stage growth and mortality of teleost fish*. Journal of Fish Biology 51(sA):52–83
Izzo C, Reis-Santos P, Gillanders BM (2018) Otolith chemistry does not just reflect environmental conditions: a meta-analytic evaluation. Fish Fish 19(3):441–454
Jonsson B, Jonsson N (1993) Partial migration: niche shift versus sexual maturation in fishes. Rev Fish Biol Fish 3(4):348–365
Kalish JM (1991) Determinants of otolith chemistry: seasonal variation in the composition of blood plasma, endolymph and otoliths of bearded rock cod Pseudophycis barbatus. Marine Ecol Prog Ser 74:137–159
Kennedy BP, Blum JD, Folt CL, Nislow KH (2000) Using natural strontium isotopic signatures as fish markers: methodology and application. Can J Fish Aquat Sci 57(11):2280–2292
Kerr L, Cadrin S, Secor D (2010) The role of spatial dynamics in the stability, resilience, and productivity of an estuarine fish population. Ecol Appl 20(2):497–507
Kerr LA, Secor DH, Piccoli PM (2009) Partial migration of fishes as exemplified by the estuarine-dependent white perch. Fisheries 34(3):114–123
Kim C-K, Park K (2012) A modeling study of water and salt exchange for a micro-tidal, stratified northern Gulf of Mexico estuary. J Mar Syst 96:103–115
Kimmerer WJ (2002) Physical, biological, and management responses to variable freshwater flow into the San Francisco estuary. Estuaries 25(6):1275–1290
Kraus RT, Secor DH (2004) Incorporation of strontium into otoliths of an estuarine fish. J Exp Mar Biol Ecol 302(1):85–106
Lankford TE, Targett TE (1994) Suitability of estuarine nursery zones for juvenile weakfish (Cynoscion regalis): effects of temperature and salinity on feeding, growth and survival. Mar Biol 119(4):611–620
Limburg KE, Wuenschel MJ, Hüssy K, Heimbrand Y, Samson M (2018) Making the Otolith magnesium chemical calendar-clock tick: plausible mechanism and empirical evidence. Rev Fish Sci Aquaculture 26(4):479–493
Livernois MC, Powers SP, Albins MA, Mareska JF (2020) Habitat associations and co-occurrence patterns of two estuarine-dependent predatory fishes. Marine Coast Fish 12(1):64–77
Loewen TN, Carriere B, Reist JD, Halden NM, Anderson WG (2016) Linking physiology and biomineralization processes to ecological inferences on the life history of fishes. Comp Biochem Physiol A Mol Integr Physiol 202:123–140
Longerich HP, Jackson SE, Gunther D (1996) Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J Anal At Spectrom 11(9):899–904
Lowe MR, DeVries DR, Wright RA, Ludsin SA, Fryer BJ (2009) Coastal largemouth bass (Micropterus salmoides) movement in response to changing salinity. Can J Fish Aquat Sci 66(12):2174–2188
Lowe MR, DeVries DR, Wright RA, Ludsin SA, Fryer BJ (2011) Otolith microchemistry reveals substantial use of freshwater by southern flounder in the northern Gulf of Mexico. Estuar Coasts 34(3):630–639
Lowerre-Barbieri SK, Barbieri LR, Flanders JR, Woodward AG, Cotton CF, Knowlton MK (2008) Use of passive acoustics to determine red drum spawning in Georgia waters. Trans Am Fish Soc 137(2):562–575
Lowerre-Barbieri SK, Tringali MD, Shea CP, Walters Burnsed S, Bickford J, Murphy M, Porch C, Durif C (2019) Assessing red drum spawning aggregations and abundance in the eastern Gulf of Mexico: a multidisciplinary approach. ICES J Mar Sci 76(2):516–529
Macdonald JI, Crook DA (2010) Variability in Sr:Ca and Ba:Ca ratios in water and fish otoliths across an estuarine salinity gradient. Mar Ecol Prog Ser 413:147–161
Martin GB, Thorrold SR, Jones CM (2004) Temperature and salinity effects on strontium incorporation in otoliths of larval spot (Leiostomus xanthurus). Can J Fish Aquat Sci 61(1):34–42
Mayr E (1926) Die Ausbreitung des Girlitz (Serinus canaria serinus L.). J Ornithol 74(3):571–671
McCormick SD (2001) Endocrine control of osmoregulation in teleost fish. Am Zool 41(4):781–794
McDonald MD, Grosell M (2006) Maintaining osmotic balance with an aglomerular kidney. Comp Biochem Physiol A Mol Integr Physiol 143(4):447–458
McMillan MN, Izzo C, Junge C, Albert OT, Jung A, Gillanders BM, Handling S editor: David(2017) Analysis of vertebral chemistry to assess stock structure in a deep-sea shark, Etmopterus spinax. ICES J Mar Sci 74(3):793–803
Miller JA (2011) Effects of water temperature and barium concentration on otolith composition along a salinity gradient: implications for migratory reconstructions. J Exp Mar Biol Ecol 405(1–2):42–52
Miller, J.A., and T.P. Hurst. 2020. Growth rate, ration, and temperature effects on Otolith elemental incorporation. Frontiers in Marine Science
Morgan JD, Sakamoto T, Grau EG, Iwama GK (1997) Physiological and respiratory responses of the Mozambique Tilapia (Oreochromis mossambicus) to salinity acclimation. Comp Biochem Physiol A Physiol 117(3):391–398
Morissette O, Lecomte F, Verreault G, Legault M, Sirois P (2016) Fully equipped to succeed: migratory contingents seen as an intrinsic potential for striped bass to exploit a heterogeneous environment early in life. Estuar Coasts 39(2):571–582
Morse JW, Bender ML (1990) Partition coefficients in calcite: examination of factors influencing the validity of experimental results and their application to natural systems. Chem Geol 82:265–277
Neill WH, Brandes TS, Burke BJ, Craig SR, Dimichele LV, Duchon K, Edwards RE, Fontaine LP, Gatlin Iii DM, Hutchins C, Miller JM, Ponwith BJ, Stahl CJ, Tomasso JR, Vega RR (2004) Ecophys.Fish: a simulation model of fish growth in time-varying environmental regimes. Rev Fish Sci 12(4):233–288
Nelson TR, DeVries DR, Wright RA (2018) Salinity and temperature effects on element incorporation of gulf killifish Fundulus grandis Otoliths. Estuar Coasts 41(4):1164–1177
Nelson TR, Powers SP (2019) Validation of species specific otolith chemistry and salinity relationships. Environ Biol Fish 102(5):801–815
Nelson TR, Powers SP (2020a) Elemental concentrations of water and Otoliths as salinity proxies in a northern Gulf of Mexico estuary. Estuar Coasts 43:843–864
Nelson TR, Powers SP (2020b) Estimates of red drum mortality via acoustic telemetry. Marine Coast Fish 12(1):78–97
Nims MK, Walther BD (2014) Contingents of southern flounder from subtropical estuaries revealed by otolith chemistry. Trans Am Fish Soc 143(3):721–731
Overstreet RM (1983) Aspects of the biology of the red drum, Sciaenops ocellatus, in Mississippi. Gulf Research Reports 7(Supplement 1):45–68
Paton C, Hellstrom J, Paul B, Woodhead J, Hergt J (2011) Iolite: freeware for the visualisation and processing of mass spectrometric data. J Anal At Spectrom 26(12):2508–2518
Phillis CC, Ostrach DJ, Ingram BL, Weber PK (2011) Evaluating otolith Sr/Ca as a tool for reconstructing estuarine habitat use. Can J Fish Aquat Sci 68(2):360–373
Porch CE (2000) Status of the red drum stocks of the Gulf of Mexico. Southeast Fisheries Science Center, Miami, FL SFD-99/00–85: 1–62
Powers S, Burns K (2010) Summary report of the red drum special working group for the Gulf of Mexico fishery management council. Tampa, FL, USA: 1–7
Powers SP, Hightower CL, Drymon JM, Johnson MW (2012) Age composition and distribution of red drum (Sciaenops ocellatus) in offshore waters of the north Central Gulf of Mexico: an evaluation of a stock under a federal harvest moratorium. Fish Bull 110(3):283–292
Pringle CM, Freeman MC, Freeman BJ (2000) Regional effects of hydrologic alterations on riverine macrobiota in the New World: tropical-temperate comparisons. BioScience 50(9):807–823
Quist MC, Pegg MA, DeVries DR (2012) Age and growth. In: Zale AV, Parrish DL, Sutton TM (eds) Fisheries techniques. American Fisheries Society, Bethesda, pp 677–721
Reis-Santos P, Vasconcelos RP, Tanner SE, Fonseca VF, Cabral HN, Gillanders BM (2018) Extrinsic and intrinsic factors shape the ability of using otolith chemistry to characterize estuarine environmental histories. Mar Environ Res 140:332–341
Reyier EA, Lowers RH, Scheidt DM, Adams DH (2011) Movement patterns of adult red drum, Sciaenops ocellatus, in shallow Florida lagoons as inferred through autonomous acoustic telemetry. Environ Biol Fish 90(4):343–360
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Can 191:382
Rohtla M, Vetemaa M (2016) Otolith chemistry chimes in: migratory environmental histories of Atlantic tarpon (Megalops atlanticus) caught from offshore waters of French Guiana. Environ Biol Fish 99(8):593–602
Rooker JR, Holt SA (1997) Utilization of subtropical seagrass meadows by newly settled red drum Sciaenops ocellatus: patterns of distribution and growth. Mar Ecol Prog Ser 158:139–149
Rooker JR, Kraus RT, Secor DH (2004) Dispersive behaviors of black drum and red drum: is otolith Sr : Ca a reliable indicator of salinity history? Estuaries 27(2):334–341
Sadovy Y, Severin KP (1992) Trace elements in biogenic aragonite: correlation of body growth rate and strontium levels in the Otoliths of the white grunt, Haemulon Plumieri (Pisces: Haemulidae). Bull Mar Sci 50(2):237–257
Sampaio LSA, Bianchini A (2002) Salinity effects on osmoregulation and growth of the euryhaline flounder Paralichthys orbignyanus. J Exp Mar Biol Ecol 269(2):187–196
Scavia D, Field JC, Boesch DF, Buddemeier RW, Burkett V, Cayan DR, Fogarty M, Harwell MA, Howarth RW, Mason C, Reed DJ, Royer TC, Sallenger AH, Titus JG (2002) Climate change impacts on U.S. coastal and marine ecosystems. Estuaries 25(2):149–164
Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS (2010) Population diversity and the portfolio effect in an exploited species. Nature 465(7298):609–612
Schroeder WW, WJWiseman Jr. (1999) Geology and Hydrodynamics of Gulf of Mexico Estuaries. In Biogeochemistry of Gulf of Mexio Estuaries, ed. T.S. Bianchi, J.R. Pennock and R.R. Twilley, 3–28: John Wiley & Sons
Secor DH (1999) Specifying divergent migrations in the concept of stock: the contingent hypothesis. Fish Res 43(1–3):13–34
Secor DH, Gunderson TE, Karlsson K (2000) Effect of temperature and salinity on growth performance in anadromous (Chesapeake Bay) and nonanadromous (Santee-Cooper) strains of striped bass Morone saxatilis. Copeia 2000(1):291–296
Secor DH, Kerr LA (2009) Lexicon of life cycle diversity in diadromous and other fishes. In Challenges for diadromous fishes in a dynamic global environment. American Fisheries Society, Symposium, 537–556
Secor DH, Rooker JR, Zlokovitz E, Zdanowicz VS (2001) Identification of riverine, estuarine, and coastal contingents of Hudson River striped bass based upon otolith elemental fingerprints. Mar Ecol Prog Ser 211:245–253
Seeley ME, Walther BD (2018) Facultative oligohaline habitat use in a mobile fish inferred from scale chemistry. Mar Ecol Prog Ser 598:233–245
Sinclair DJ, Kinsley LPJ, McCulloch MT (1998) High resolution analysis of trace elements in corals by laser ablation ICP-MS. Geochim Cosmochim Acta 62(11):1889–1901
Smith WD, Miller JA, Heppell SS (2013) Elemental markers in elasmobranchs: effects of environmental history and growth on vertebral chemistry. PLoS One 8(10):e62423
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci 60(3):1129–1157
Stunz GW, Minello TJ, Levin PS (2002) Growth of newly settled red drum Sciaenops ocellatus in different estuarine habitat types. Mar Ecol Prog Ser 238:227–236
Sturrock AM, Hunter E, Milton JA, Johnson RC, Waring CP, Trueman CN, Leder E (2015) Quantifying physiological influences on otolith microchemistry. Methods Ecol Evol 6(7):806–816
Sturrock AM, Trueman CN, Darnaude AM, Hunter E (2012) Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? J Fish Biol 81(2):766–795
Sturrock AM, Trueman CN, Milton JA, Waring CP, Cooper MJ, Hunter E (2014) Physiological influences can outweigh environmental signals in otolith microchemistry research. Mar Ecol Prog Ser 500:245–264
Tipsmark CK, Luckenbach JA, Madsen SS, Kiilerich P, Borski RJ (2008) Osmoregulation and expression of ion transport proteins and putative claudins in the gill of southern flounder (Paralichthys lethostigma). Comp Biochem Physiol A Mol Integr Physiol 150(3):265–273
Townsend DW, Radtke RL, Corwin S, Libby DA (1992) Strontium: calcium ratios in juvenile Atlantic herring Clupea harengus L. otoliths as a function of water temperature. J Exp Mar Biol Ecol 160(1):131–140
Tupitza JC, Glaspie CN (2020) Restored freshwater flow and estuarine benthic communities in the northern Gulf of Mexico: research trends and future needs. PeerJ 8:e8587
VanderKooy S (2009) A practical handbook for determining the ages of Gulf of Mexico fishes second edition. Gulf States Marine Fisheries Commission 167:1–157
Walther BD, Kingsford MJ, O’Callaghan MD, McCulloch MT (2010) Interactive effects of ontogeny, food ration and temperature on elemental incorporation in otoliths of a coral reef fish. Environ Biol Fish 89(3):441–451
Walther BD, Limburg KE (2012) The use of otolith chemistry to characterize diadromous migrations. J Fish Biol 81(2):796–825
Walther BD, Thorrold SR (2006) Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish. Marine Ecol Prog Ser 311:125–130
Watson CJ, Nordi WM, Esbaugh AJ (2014) Osmoregulation and branchial plasticity after acute freshwater transfer in red drum, Sciaenops ocellatus. Comp Biochem Physiol A Mol Integr Physiol 178:82–89
Webb SD, Woodcock SH, Gillanders BM (2012) Sources of otolith barium and strontium in estuarine fish and the influence of salinity and temperature. Mar Ecol Prog Ser 453:189–199
Wilson CA, Nieland DL (1994) Reproductive biology of red drum, Sciaenops ocellatus, from the neritic waters of the northern Gulf of Mexico. Fish Bull 92(4):841–850
Winner BL, Flaherty-Walia KE, Switzer TS, Vecchio JL (2014) Multidecadal evidence of recovery of Nearshore red drum stocks off west-Central Florida and connectivity with inshore nurseries. N Am J Fish Manag 34(4):780–794
Zlokovitz ER, Secor DH, Piccoli PM (2003) Patterns of migration in Hudson River striped bass as determined by otolith microchemistry. Fish Res 63(2):245–259
Acknowledgments
We thank the crew from Raffield Fisheries for their help with collection of adult Red Drum from schools. Andrea Kroetz, Lindsey Lachenmyer, Tom Guoba, Claire Pabody, Pearce Cooper, Meagan Schrandt and other students, interns, and technicians with the Fisheries Ecology Lab (University of South Alabama / Dauphin Island Sea Lab) are thanked for their help with Red Drum collection and sample processing. Assistance with LA-ICPMS analysis at the DISL instrumentation lab was provided by Laura Linn and she is thanked for her help. Funding for this work was provided by the NOAA Saltonstall-Kennedy Grant Program (NA14NMF4270046) and the National Fish and Wildlife Foundation, Gulf Environment Benefit Fund via a subcontract from the Alabama Department of Conservation and Natural Resources. This project was reviewed and approved (Protocol #280269) by the Institutional Animal Care and Use Committee at the University of South Alabama. Finally we thank anonymous reviewers for their comments, which have greatly improved this manuscript.
Availability of data and material
Elemental ratio data from all Red Drum can be provided from the corresponding author if requested and is plotted for transparency in Online Resources 1-3.
Funding
This study was funded through the NOAA Saltonstall-Kennedy Grant Program (NA14NMF4270046) and through the National Fish and Wildlife Foundation’s Gulf Environment Benefit Fund via a sub-contract from the Alabama Department of Conservation and Natural Resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No authors have any conflict of interest.
Ethics approval
This project was reviewed and approved (Protocol #280269) by the Institutional Animal Care and Use Committee at the University of South Alabama.
Code availability
All analyses were completed in R and the code can be provided from the corresponding author if requested.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Online Resource 1
Lifetime Sr:Ca ratios of oligohaline resident Red Drum during years 2 and 3 of life (≥ 90% of these years spent in oligohaline waters). The mean oligohaline threshold value was used for classification (horizontal dashed lines) and the gray shaded box represents the range between low and high thresholds. Fish age is on the x-axis of each panel and tick marks correspond with measured annuli locations (PDF 239 kb)
Online Resource 2
Lifetime Sr:Ca ratios of all Red Drum that spent years 2 and 3 of life in oligohaline and meso-polyhaline salinities (≥10% and < 90% in oligohaline waters). The mean oligohaline threshold value was used for classification (horizontal dashed lines) and the gray shaded box represents the range between low and high thresholds. Fish age is on the x-axis of each panel and tick marks correspond with measured annuli locations (PDF 471 kb)
Online Resource 3
Lifetime Sr:Ca ratios of all meso-polyhaline resident Red Drum during years 2 and 3 of life (< 10% of these years spent in oligohaline waters). The mean oligohaline threshold value was used for classification (horizontal dashed lines) and the gray shaded box represents the range between low and high thresholds. Fish age is on the x-axis of each panel and tick marks correspond with measured annuli locations (PDF 380 kb)
Online Resource 4
von Bertalanffy growth models fit to predicted length at age. Corrected Akaike information criterion (AICc) and the ΔAICc (model AICc – lowest AICc). The g notation indicates specific parameters in each model that varied across sex or salinity groups (oligohaline resident vs. non-resident). Low, mean, and high correspond to models run with Red Drum classified as either oligohaline residents or not, during years 2–3 of life, across these three oligohaline threshold values (DOCX 14 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nelson, T.R., Hightower, C.L., Coogan, J. et al. Patterns and consequences of life history diversity in salinity exposure of an estuarine dependent fish. Environ Biol Fish 104, 419–436 (2021). https://doi.org/10.1007/s10641-021-01080-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01080-0