Abstract
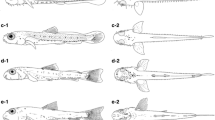
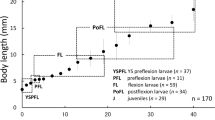
Morphological development, including the body proportions, fins, pigmentation and labyrinth organ, in laboratory-hatched larval and juvenile three-spot gourami Trichogaster trichopterus was described. In addition, some wild larval and juvenile specimens were observed for comparison. Body lengths of larvae and juveniles were 2.5 ± 0.1 mm just after hatching (day 0) and 9.2 ± 1.4 mm on day 22, reaching 20.4 ± 5.0 mm on day 40. Aggregate fin ray numbers attained their full complements in juveniles >11.9 mm BL. Preflexion larvae started feeding on day 3 following upper and lower jaw formation, the yolk being completely absorbed by day 11. Subsequently, oblong conical teeth appeared in postflexion larvae >6.4 mm BL (day 13). Melanophores on the body increased with growth, and a large spot started forming at the caudal margin of the body in flexion postlarvae >6.7 mm BL, followed by a second large spot positioned posteriorly on the midline in postflexion larvae >8.6 mm BL. The labyrinth organ differentiated in postflexion larvae >7.9 mm BL (day 19). For eye diameter and the first soft fin ray of pelvic fin length, the proportions in laboratory-reared specimens were smaller than those in wild specimens in 18.5–24.5 mm BL. The pigmentation pattern of laboratory-reared fish did not distinctively differ from that in the wild ones. Comparisons with larval and juvenile morphology of a congener T. pectoralis revealed several distinct differences, particularly in the numbers of myomeres, pigmentations and the proportional length of the first soft fin ray of the pelvic fin.






Similar content being viewed by others
References
Burggren WW (1979) Bimodal gas exchange during variation in environmental oxygen and carbon dioxide in the air breathing fish Trichogaster trichopterus. J Exp Biol 82:197–213
Burggren W, Haswell S (1979) Aerial CO2 excretion in the obligate air breathing fish Trichogaster pectoralis: a role for carbonic anhydrase. J Exp Biol 82:215–225
Cole B, Tamaru CS, Bailey R, Brown C (1999) A manual for commercial production of the gourami, Trichogaster trichopterus, a temporary paired spawner. Center for Tropical and Subtropical Aquaculture, Publ No 135, Oahu
Iwata A, Ohnishi N, Kiguchi Y (2003) Habitat use of fishes and fishing activity in plain area of southern Laos. Asian and African Area Studies 3:51–86
Kohno H (1998) Early life history features influencing larval survival of cultivated tropical finfish. In: De Silva SS (ed) Tropical mariculture. Academic Press, London, pp 71–111
Kottelat M (1998) Fishes of the Nam Theun and Xe Bangfai basins, Laos, with diagnoses of twenty-two new species (Teleostei: Cyprinidae, Balitoridae, Cobitidae, Coiidae and Odontobutidae). Ichthyol Explor Freshwat 9:1–128
Kottelat M (2001) Fish of Laos. Gunaratne Offset Ltd, Colombo
Kramer DL (1973) Parental behaviour in the blue gourami Trichogaster trichopterus (Pisces, Belontiidae) and its induction during exposure to varying numbers of conspecific eggs. Behavior 47:14–32
Leis JM, Trnski T (1989) The larvae of Indo-Pacific shorefishes. NSW University Press, Kensington
Lever C (1996) Naturalized fishes of the world. Academic Press, California, USA
Matayoshi S, Shokita S (1982) Development of air-breathing organ in Paradicefish, Macropodus opercularis (Linnaeus) from Okinawa-jima, the Ryukyus. Bull Coll Sci Univ Ryukyus 33:61–67
Matsumiya Y, Kanamaru H (1987) Morphometric comparison and differentiation between artificially-released and wild red sea bream (Pagrus major). J Appl Ichthyol 3:49–55
Matsumoto S, Morioka S, Kumagai S (2001) Development of African catfish Clarias gariepinus larvae during the transitional phase between endogenous and exogenous energy intake. In: Weyl OLF, Weyl MV (eds) Proceedings of the Lake Malawi Fisheries Management Symposium. Department of Fisheries of Malawi, Lilongwe, pp 227–232
Morioka S, Vongvichith B (2011) Growth and morphological development of laboratory-reared larval and juvenile Hemibagrus filamentus (Siluriformes: Bagridae). Ichthyol Res 58:245–254
Morioka S, Ito S, Kitamura S, Vongvichith B (2009) Growth and morphological development of laboratory-reared larval and juvenile climbing perch Anabas testudineus. Ichthyol Res 56:162–171
Morioka S, Ito S, Kitamura S (2010a) Growth and morphological development of laboratory-reared larval and juvenile snakeskin gourami Trichogaster pectoralis. Ichthyol Res 57:24–31
Morioka S, Sano K, Phommachan P, Vongvichith B (2010b) Growth and morphological development of laboratory-reared larval and juvenile Pangasianodon hypophthalmus. Ichthyol Res 57:139–147
Moteki S, Yoseda K, Sahin T, Üstündağ C, Kohno H (2001) Transition from endogenous to exogenous nutritional sources in larval Black Sea turbot Psetta maxima. Fish Sci 67:571–578
Na-Nakorn U, Kamonrat W, Ngamsiri T (2004) Genetic diversity of walking catfish, Clarias macrocephalus, in Thailand and evidence of genetic introgression from introduced farmed C. gariepinus. Aquaculture 240:145–163
Ogata Y, Morioka S, Sano K, Vongvichith B, Eda H, Kurokura H, Khonglaliane T (2010) Growth and morphological development of laboratory-reared larvae and juveniles of the Laotioan indigenous cyprinid Hypsibarbus malcolmi. Ichthyol Res 57:389–397
Rainboth WJ (1996) Fishes of the Cambodian Mekong. FAO Species Identification Field Guide for Fishery Purposes. FAO, Rome
Rüber L, Britz R, Zardoya R (2006) Molecular phylogenetics and evolutionary diversification of labyrinth fishes (Perciformes: Anabantoidei). Syst Biol 55:374–397
Senanan W, Kapuscinski AR, Na-Nakorn U, Miller LM (2004) Genetic impacts of hybrid catfish farming (Clarias macrocephalus × C. gariepinus) on native catfish populations in central Thailand. Aquaculture 235:167–184
Shimizu H, Shiozawa S (2004) Allometry and development of caudal skeleton of hatchery-reared yellowfin tuna Thunnus albacares. Bull Fish Res Agen 10:1–7
Welcomme RL (1988) International introductions of inland aquatic species. FAO Fisheries Technical Paper 294, Rome
Welcomme RL, Vidthayanon C (2003) The impact of introductions and stocking of exotic species in the Mekong basin and policies for their control. Technical Paper No 9, Mekong River Commission, Phnom Penh
Acknowledgments
We express our sincere gratitude to K. Sano, Fisheries and Aquaculture International Co., Ltd., and the staff of the Aquaculture Improvement and Extension Project of the Japan International Cooperation Agency for their kind cooperation on broodstock collection. We are also grateful to L. Khamsivilay, Living Aquatic Resources Research Center, Laos, for his logistical support. Our thanks also go to G. Hardy for his polite and constructive English revision.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Morioka, S., Chanthasone, P., Phommachan, P. et al. Growth and morphological development of laboratory-reared larval and juvenile three-spot gourami Trichogaster trichopterus . Ichthyol Res 59, 53–62 (2012). https://doi.org/10.1007/s10228-011-0256-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-011-0256-9