Abstract
The capacity to navigate by layout geometry has been widely recognized as a robust strategy of place-finding. It has been reported in various species, although most studies were performed with vision-based paradigms. In the presented study, we aimed to investigate layout symmetry-based navigation in the house cricket, Acheta domesticus, in the absence of visual cues. For this purpose, we used a non-visual paradigm modeled on the Tennessee Williams setup. We ensured that the visual cues were indeed inaccessible to insects. In the main experiment, we tested whether crickets are capable of learning to localize the centrally positioned, inconspicuous cool spot in heated arenas of various shapes (i.e., circular, square, triangular, and asymmetric quadrilateral). We found that the symmetry of the arena significantly facilitates crickets’ learning to find the cool spot, indicated by the increased time spent on the cool spot and the decreased latency in locating it in subsequent trials. To investigate mechanisms utilized by crickets, we analyzed their approach paths to the spot. We found that crickets used both heuristic and directed strategies of approaching the target, with the dominance of a semi-directed strategy (i.e., a thigmotactic phase preceding direct navigation to the target). We propose that the poor performance of crickets in the asymmetrical quadrilateral arena may be explained by the difficulty of encoding its layout with cues from a single modality.
Similar content being viewed by others
Introduction
Spatial navigation plays a vital role in the lives of the animals, allowing them to successfully forage for food, find their way back to nests, or localize mating spots. To this end, animals employ a spectrum of strategies that allow them to repeatedly return to memorized locations despite the constantly changing environment and disrupting stimuli (Gallistel 1990; Thinus-Blanc et al. 2010; Tommasi et al. 2012). One of the strategies enabling the mitigation of the constant change of environmental features is to navigate by the relational pattern of the objects in the surrounding space (layout geometry) instead of by their particular features. Those relations could be perceived via one or more modalities (Cheung et al. 2008; Stürzl et al. 2008; Webb and Wystrach 2016; Buehlmann et al. 2020). Nevertheless, the contribution of vision seems to be investigated much more than any other modality, constituting a somewhat “visiocentric” bias in spatial navigation studies (Hohol et al. 2017). However, the capacity for processing geometric relations should be feature-independent, as these relations are preserved across the modalities (Gallistel 1990; Duval 2019). In this regard, we want to highlight the importance of studies that are focused on non-visual modalities. This approach follows rationales similar to the cross-modal testing of abstract representations (Izard et al. 2009; Dehaene 2011; Butterworth 2022; Nieder 2017; Giurfa 2013; Giurfa et al. 2001).
The body of research on animals’ navigation by layout symmetry consists primarily of data obtained from vertebrates. It was found that using layout geometry as a cue for navigation is not task-dependent since vertebrates are able to localize the center of an arena based on its overall geometric shape, and they are capable of transferring this knowledge to other geometrically regular enclosures (Tommasi et al. 1997; Gray et al. 2004; Tommasi and Thinus-Blanc 2004; Tommasi and Giuliano 2014). Localizing the center based on discrete landmarks has also been investigated (Kamil and Jones 1997, 2000; Sutton et al. 2000; Potì et al. 2010). Research on insect navigation utilizing environmental geometry is scarce, and mainly concerns navigation in rectangular arenas (Wystrach and Beugnon 2009; Sovrano et al. 2012; Lee and Vallortigara 2015). However, aside from the studies directly concerning layout geometry, there are other reports suggesting that miniature nervous systems are able to process the geometric properties of objects, such as symmetry (Giurfa et al. 1996; Møller and Sorci 1998; Rodríguez et al. 2004; White and Kemp 2020).
While there is still no consensus about the exact mechanism responsible for the observed behavioral patterns of layout geometry-driven navigation in vertebrates (Cheng et al. 2013; Sutton and Newcombe 2014; Duval 2019; Hohol 2020), in the case of insects, it is widely accepted that view matching (VM) is the core mechanism behind this mode of spatial navigation (Wehner et al. 1996; Collett and Rees 1997; Judd and Collett 1998; Wystrach et al. 2011; Wystrach and Graham 2012b, a; Collett et al. 2013; Webb 2019). In brief, the VM approach assumes that the animal records a view of the area surrounding the goal and then moves so as to minimize the discrepancy between the recorded and the actual view. The memorized “view” is not simply understood as a mental image but instead as a set of encoded parameters including depth, motion, edges, or specific features, e.g., skyline and fractional position of mass (Möller 2001; Collett and Collett 2002; Zahedi and Zeil 2018; Graham and Cheng 2009; Lent et al. 2013).
Geometry-driven navigation may be constituted by a range of perceptual and cognitive mechanisms, depending on the species (Vallortigara 2018). For instance, Gigantiops destructor, a neotropical formicine ant tested by Wystrach and Beugnon (2009) for the presence of rotational errors, is an animal highly dependent on vision. Therefore, the VM-based explanation of its navigational behavior in geometrically regular arenas is convincing. Nevertheless, this does not automatically imply that the navigation of other insects, such as adult house crickets, which are predominantly nocturnal animals (Cymborowski 1973; Górska-Andrzejak and Wojtusiak 2003), in the aforementioned enclosures would also be driven by VM. Although geometry is considered mainly as a vision-based phenomenon, it has been demonstrated that geometric cognition in various species (chickens, rats, bees, spiders, humans, cavefish), both regarding objects (Marlair et al. 2021), and layouts (Sovrano et al. 2018, 2020; Nardi et al. 2021), could be grounded also in other modalities, e.g., proprioceptive and kinesthetic (Alary et al. 2008; Sguanci et al. 2010; Marlair et al. 2021). Aside from navigation by visual cues (Doria et al. 2019), crickets have been tested in experiments involving auditory information (Reeve and Webb 2003; Poulet and Hedwig 2005). Nevertheless, none of the existing studies allow us to infer their ability to use non-visually perceived layout geometry for spatial learning and navigation.
In the present study, we tested whether house crickets (Acheta domesticus) are able to find a target positioned centrally in an arena in the absence of visual cues, and if so, whether the symmetry of the spatial layout facilitates the learning of such a task. To this end, we employed a variant of the center-finding paradigm, where the animal is required to find an inconspicuous cool spot positioned at the center of the following experimental enclosures: circular, square, equilateral triangular, and asymmetric quadrilateral. Originally, the task was developed by Tommasi et al. (1997) to test the spatial cognition of chickens; later it was used to investigate other vertebrate species, namely, pigeons (Gray et al. 2004), rats (Tommasi and Thinus-Blanc 2004), and human children (Tommasi and Giuliano 2014).
Aside from exploring possible vision-independent geometric navigation, the non-visual testing conditions meet the ecological validity standard, as house cricket imagoes are predominantly active at night (Cymborowski 1973; Górska-Andrzejak and Wojtusiak 2003). As the VM generally explains navigational behavior in a low-level way, namely, the overall encoding of the layout geometry is not required, we prevented the insects from using view-based place finding. For this purpose, we employed a non-visual paradigm modeled on the Tennessee Williams (TW) setup (Mizunami et al. 1998; Wessnitzer et al. 2008; Ofstad et al. 2011), which is a “dry” analog of the Morris (1981) water maze (MWM) test commonly used for assessing navigational capabilities. The unavailability of visual cues was assessed prior to the main experiment by testing the occurrence of antennal positioning reflex towards the looming stimulus under the illumination used for the center-finding assessment. Moreover, for further confirmation of the non-visually driven nature of observed effects, center-finding in crickets with enamel-covered eyes was tested. Additionally, measures were taken to prevent the potential influence of olfactory and auditory stimuli, which could provide insects with cues for reorientation.
We expected to find that the behavior observed in this study would converge with those previously reported in vertebrates (Tommasi et al. 1997; Gray et al. 2004; Tommasi and Thinus-Blanc 2004; Tommasi and Giuliano 2014). First of all, we hypothesized that crickets would be able to learn how to find the centrally located cool spot. Since geometrically regular layouts are easier to navigate, our second hypothesis was that crickets would learn how to find the center more efficiently in conditions with symmetric arenas (circular, square, and triangular) compared to the asymmetric quadrilateral one.
Materials and methods
Experimental setup
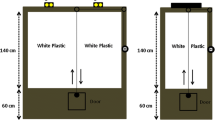
In the present study, the center-finding paradigm (Tommasi et al. 1997) was combined with the non-visual variation of the TW setup (Wessnitzer et al. 2008). The experimental apparatus (Figs. 1, 2) consisted of a leveled, matted, white glass sheet and variously shaped arenas (circular, square, triangular and asymmetric quadrilateral), all of the same height (25 cm) and adjusted to approximately the same area (709 ± 3% cm2—circular d = 30, square: edges length a = 27, triangular: edges length a = 40 asymmetric quadrilateral: edges lengths a = 37, b = 24, c = 23, d = 26, angles widths α = 67°, β = 80°, γ = 100°, δ = 113°cm). The arenas were made of solvent-welded white lucite and devoid of any visual cues. Solvent welds were utilized to ensure that corners did not provide any attachment point for the crickets. Thus, even if an insect did lean against a corner, the moment was brief as the insect slid back inside the arena. The surface of the glass was uniformly heated to 50 ± 1 °C with IR heating lamps (4 × 250 W bulbs, heat distribution was evaluated with the FLIR T640 thermal camera) with the exception of a centrally (at the geometric center in the symmetrical figures and at the centroid in the case of the quadrilateral one, designated by the intersection of the medians of the figure) localized cool spot that was maintained at a constant temperature of 25 ± 1 °C, preferred by A. domesticus (Lachenicht et al. 2010), by a water-cooling block (⌀60 mm). Exterior parts of the arenas were covered with aluminum foil to ensure that the walls were at least partially heated. All experimental trials were performed in a soundproofed dark room. Arenas were illuminated with a red LED ring (24 × WS2812B) emitting red light with a wavelength of 620–625 nm, which is below the detection threshold of the retinal receptors of crickets (Herzmann and Labhart 1989), and thus was chosen to ensure the lack of visual cues. Before each trial, the enclosures of the arenas were rotated by 45°, and the whole setup was thoroughly wiped with 70% (v/v) denatured ethyl alcohol to eliminate any olfactory clues.
The applied experimental setup iterates the TW setup, namely a spatial learning task similar to the MWM, where the insect explores a plate heated to an aversive temperature in order to find an inconspicuous cool spot on which it can rest. Our apparatus consisted of 4 × 250 W dimmable heating lamps with a fiberglass cloth diffuser mounted above them. Thermal radiation generated by the lamps uniformly heated the bottom side of the glass plate (500 × 500×4 mm), painted with two layers of heat-resistant enamel. The first layer was made of white enamel (to provide a contrasting background for insect tracking), and the second was painted black (for thermal absorption). On the backside of the plate, a 3D-printed water cooler (⌀60 mm) was attached with a Gecko pad and connected to a continuous flow of cool water, adjusted to ensure the constant temperature of a cool spot on the surface of the plate. Glass was chosen due to its low thermal conductivity, which allowed for the creation of a sharp thermal boundary around the cool spot. The upper surface of the glass was matted to ensure sufficient traction for the insects. Arena enclosures of various shapes were placed on the surface of the glass. The setup was calibrated with the aid of thermal imaging to provide stable temperatures of 50 ± 1 °C on the hot part and 25 ± 1 °C on the cool spot (see Fig. 2). Prior to every training session, the setup was warmed to the desired temperatures. For each arena shape, 15 crickets were tested (n = 15 × 4)
Thermal imaging of the heat distribution on the surfaces of the arenas. Two measurement points, pkt1 and pkt2, indicate the temperatures of the cool spot and the rest of the arena, respectively. Images were acquired with a FLIR Systems AB high-performance thermal camera FLIR T640 (640 × 480px IR resolution, sensitivity 0.04 ℃ at 30 ℃)
Animal husbandry
House crickets (Acheta domesticus, wild type) used in the study were acquired from a stock colony maintained at the Institute of Biology, Biotechnology and Environmental Protection of the University of Silesia in Katowice. Insects were reared under constant conditions of 30 ± 2 °C, 40 ± 10% RH, and a 12:12 light:dark photoperiod with water and food pellets ad libitum. In all trials, healthy adult (1–2 days after the imaginal molt) male crickets were used. The reason for using only males stems from an attempt to avoid excessive behavioral variance resulting from oviposition performed by females, as well as known burrow choice and construction exhibited by singing males from species of Gryllidae family which could indirectly indicate sensitivity to the layout structure. After conducting experimental assessment crickets were discarded to a separate retirement colony, so that each individual participated only once in the experiments.
Confirmation of inaccessibility of visual cues
To confirm the inaccessibility of the visual cues in the main experiment, we employed a two-way approach. Firstly, we ensured that the used setup sufficiently suppressed the usage of the visual cues. To this end, we conducted a test of visual behavior per se (antennal positioning reflex) under the same illumination used during the main experiment (Fig. 3). Secondly, we conducted an additional test with blinded insects (eyes carefully painted over with opaque blue Edding 751 paint marker) using exactly the same procedure and conditions as in the main experiment.
Light
To confirm that red illumination, utilized in the main experiment, was imperceptible, and that the visual cues were unavailable to insects navigating in the TW setup, we utilized the well-known antennal positioning reflex towards the looming stimuli (Yamawaki and Ishibashi 2014). The tests were conducted in two variants: the first was under the red illumination used for the main experiment, and the second was under white light. The tethered insect was positioned on a smooth Lucite disc (⌀ 12 cm, acting as an omnidirectional treadmill, allowing the insect to move its legs but without providing sufficient traction for walking), and the circular looming stimulus was presented to its left side. The movement of the antennae was recorded with an infrared camera and subsequently analyzed in BehaView (v. 0.0.23; Boguszewski 2022).
Blinded crickets
To further confirm the non-visual character of observed behavior, we conducted an additional control with blinded crickets. A day before the learning procedure (conducted exactly the same way as in the main experiment), the crickets were anesthetized with CO2, and their eyes were carefully painted over with Edding 751 paint marker (Fig. 4). This procedure did not damage the eye but rendered crickets entirely unreactive to visual stimuli.
Center finding
Prior to the experiments, each insect was removed from the general colony and underwent an initial habituation in order to familiarize it with the transfer container (black film roll case − ⌀30, h50 mm). After that, the insect was placed on the arena and left undisturbed for a 5 min trial (Fig. 5) while a recording was captured. Between subsequent trials, the cricket was removed from the apparatus for a 5 min rest. Each session consisted of 10 trials (as in Wessnitzer et al. 2008) per arena. The procedure of each trial was analogous to a typical MWM test. The cricket was released at a random location in the arena and had to find the centrally localized, inconspicuous cool spot that allowed it to escape the aversive heat stimuli. Each insect was trained on a single arena shape. Throughout the experiment, the tested arena shapes were shuffled between the days over which the recordings were collected.
Overview of the entire study. The learning procedure was based on a study by Wessnitzer et al. (2008) consisting of 10 × 5-min-long trials alternating with 5 min rests. The duration of the rest time was adjusted from the 2 min used by Wessnitzer et al. (2008) in order to reduce elevated erratic escape behavior, which was observed in a pilot study conducted prior to the primary experiment
Spontaneous activity
The assessment of the spontaneous movement of crickets in all the tested arena shapes (per shape n = 15, Σn = 120) was performed to obtain data on how likely the crickets would be to spontaneously visit the arena center. The tests were performed in two variants: firstly, with the arena heated to the same temperature as during the main experiment, and secondly, without the heating (arena’s surface was at room temperature). In both conditions, the cool spot was absent.
Data acquisition and processing
All recordings were captured with a Microsoft LifeCam Studio webcam and VirtualDub (v. 1.10.4.35491) software. Movement trajectories of tested insects were extracted with SwisTrack 4 software (Lochmatter et al. 2008) and further processed in RStudio (v. 4.0.1, R Core Team 2021) with the trajr package (McLean and Skowron Volponi 2018). To obtain detailed data on the strategies that the insects used to navigate to the center, the DeepLabCut 2.0 (Mathis et al. 2018), machine learning-based tracking framework was used. In each video, all corners (or in the case of the circle, four furthermost points on the arena’s perimeter, at opposite ends of two perpendicular diameters) were tracked along with insect position. This allowed the analysis of insect movement in the spatial context of the arena.
Statistical analysis
Statistical data analyses were performed in the R software for statistical computing (v. 4.0.1, R Core Team 2021) and GraphPad Prism 9 software. To test whether crickets showed any signs of learning to find the cool spot at arena centers, we used mixed-effects linear regression models (LMMs), utilizing the lme4 (Bates et al. 2015) and lmerTest (Kuznetsova et al. 2017) R-packages. Firstly, we tested the change in the proportion of time spent on the centrally located cool spot throughout time (trial repetitions) in each arena. The proportion of time spent in each arena center was used as a response variable, which was square-root-transformed prior to model fitting to normalize the variable’s distribution. Time (trial number) and arena shape were used as predictors, also included was the interaction between them, i.e., the ability to estimate a learning curve for each arena type. Secondly, we tested the change in latency until the first arrival at the cool spot throughout time; in this model, time passed between the start of the trial and the cricket finding the cool spot was the response variable (log transformed to normalize its distribution), and similarly to the previously described model, time, arena shape, and their interaction were specified as predictors. In both LMMs, we used the ID of individual crickets as a random effect, and also controlled for between-individual variation in slope estimates (random intercept and slope LMMs).
To test whether or not the proportion of time spent at the arena's center (i.e., at the cool spot) by crickets differs significantly between the treatment (heated arena, with the cool spot in the center) and control (non-heated arena) groups, during the first trial, we used Mann–Whitney’s test. This meant carrying out five Mann–Whitney tests between control and treatment groups for the circle, square, triangle, and quadrilateral arenas, as well as for the circular arena with blinded crickets. Since we used multiple comparison tests on the same dataset, we applied Bonferroni’s post hoc P value adjustment on the P values from the Mann–Whitney tests, to reduce the probability of type I errors.
A comparison of insects’ spontaneous activity was performed using GraphPad Prism 9, which was also used for preparation of corresponding plots. Statistical analysis consisted of two-way ANOVA, with Šidák’s multiple comparisons test.
To investigate the strategies that were utilized to reach the cool spot, we analyzed trajectories preceding successfully locating the cool spot (a stay lasting for at least 5 s spent on the cool spot). For the comparison, all tracking data were aligned so that the position of the cool spot was set at 0, 0, with all other coordinates (including the insect position and the arena geometry location) recalculated accordingly. The trajectories from all trials in all arenas were analyzed, and their numerosities were counted. Each approach path, consisting of insect coordinates in every video frame during the last three seconds preceding the successful stay, was isolated. Paths with less than 5% time spent outside the cool spot (movement on the cool spot edge) were excluded to minimize noise.
For each frame, the distance of insect to the arena perimeter was evaluated. For this purpose, we employed a WKT geometry description and the methods from the rgeos package (Bivand and Rundel 2021). To compare the dynamics of approaches in all arenas, we used the Cartesian distance from each arena’s perimeter (Fig. 6). We calculated the via function gDistance from the aforementioned package. Collected wall distance time series were feature-scaled (min–max normalization in the range [0–1]—the minimal values were calculated as 0 and maximal to 1) and analyzed using dynamic time warping (algorithm measuring the similarity of time series, which may vary in speed) to extract the clusters of similar approaches (Müller 2007). On the basis of optimization of clustering performance metrics available in the package dtwclust (Sardá-Espinosa 2019) as well as the observation of data, it was decided to set the number of clusters at k = 4. For visualization purposes, the trajectories corresponding to the clusters’ medoids (the most centrally located instance in the cluster) were used (colored red in the figures).
Results
Confirmation of inaccessibility of visual cues
The presence of antennal positioning reflex towards the looming stimuli starkly differed, depending on the illumination under which the crickets were tested (Fig. 7). Under the white illumination, the reflex was exhibited by the majority of tested crickets, while under the red (used in the main experiment) illumination, it was mostly absent, thus corroborating the inaccessibility of visual cues in the experimental setup.
Spontaneous activity
Patterns of spontaneous spatial exploration (Fig. 8), as indicated by the percentage of time spent in proximity of the walls, and spent in a centrally located spot (corresponding to the position of the cool spot in the main experiment), indicate significant differences in the exploration of various layouts. Additionally, the percentage of total time spent resting differed significantly between the circle-shaped arena and other layouts, representing the most intensive exploration of the circular arena. Considering the aims of the study, the observable differences point toward a strong thigmotaxis behavior and a strong avoidance of the central region of the arenas (especially when the surface is heated, as during the main experiment), indicating the lack of initial preference of the center of any arena shape used in the main experiment.
Movement characteristics for a range of arena shapes. a distance travelled, b time in rest, c wall following time, d time on spot. Values are presented as mean and SD. Letters indicate statistically significant differences between arenas: small letters for cold and big letters for hot conditions, *statistically significant differences between conditions for particular arena shape. Two-way ANOVA Šidák’s multiple comparisons test, p < 0.05, N = 15
Center finding learning
In the trials, crickets tended to spend more time at the cool spot in all the symmetric arenas, but not in the asymmetric quadrilateral one (Fig. 9 and Table 1, Part A). We found no significant differences in the learning curve slopes between the circular, triangular, and square arenas. However, we found that the learning curve estimate (slope) was an order of magnitude lower in the asymmetric arena. Furthermore, we found that crickets’ latency in finding the arena centers significantly decreased in all arena types (Fig. 9 and Table 1, Part B). Arena type differences in the slopes of the latency-reduction were only found between the triangular arena versus the square, quadrilateral, and circular ones, as the slope of the estimated regression line was steepest in the former.
Regression slope estimates from the LMM on the time proportion spent on the cool spot (upper left) and on the latency until arriving at the arena centers (lower left) by crickets: letters denote the significance of arena type differences in slope estimates; asterisks mark slope estimates significantly greater than zero. Regression lines are also visualized for the time proportion spent on the cool spot (upper right) and on the latency until arriving at the arena centers (lower right) as a function of time (trials). Error bars (blue) represent the 95% confidence intervals around the slope estimates
Comparison of spontaneous exploration and performance in the first trial
Crickets spontaneously exploring unheated arenas spent significantly less time at the arena center than all groups during the first trial (Fig. 10) circle (blind) (P < 0.001), circle (P < 0.001), triangle (P = 0.027), square, although the latter was only marginally significant after Bonferroni’s P value adjustment (P = 0.079), with the exception of crickets navigating a quadrilateral arena (P = 1).
Approach path characteristics
In total, throughout the main experiment, crickets performed 1349 successful approaches to the cool spot; out of those, based on similarity, four clusters were isolated and characterized (Fig. 11).
Approach path numerosities in each cluster grouped by arena shapes reveal that in all arena shapes, the cluster 4-type approach paths were used the most frequently, followed by cluster 1 type (Fig. 12). This pattern prevails even in the quadrilateral arena, with the lowest number of successful approaches made in total (which correspond to the observed lowest percentage of time spent on the cool spot and the highest latency to locate it). The second, less-pronounced variation from the overall pattern is a slight elevation of number of cluster 3 type approaches in the circular arena.
Trajectories preceding finding the centrally located cool spot from circular arena representative (medoids) for each cluster. General descriptive characteristic of cluster members: cluster 1: gradual approach towards the center, both in diagonally oriented trajectories and in a spiral-like mode, cluster 2: fast, archlike ventures towards the center and back, towards the perimeter, cluster 3: ventures from the center towards the walls, and back again, cluster 4: wall-following and direct approach to center
Discussion
In the present study, we aimed to test whether the insects could learn to successfully navigate without visual cues, relying only on layout symmetry perceived tactilely. Additionally, we attempted to characterize the strategies allowing insects to navigate successfully, with particular attention to differentiating between heuristic ones (independent of knowledge about layout acquired by the individual) and those that relied on perception and memory of environmental layout. To this end, we employed a non-visual version of the center-finding paradigm with additional tests, thus ensuring the inaccessibility of visual cues in experimental conditions. While we are aware that the task used in the study is exceedingly simplistic in comparison to the natural environments in which insects have to navigate in their daily life, our goal was to ensure maximal separation from the stimuli that could overshadow the possible presence of geometry-based navigation capacities in our model species.
Despite the lack of previous insect research conducted with the center-finding paradigm, our hypotheses were driven by the general theoretical claim that sensitivity to layout geometry increases the environmental adaptation of the animals. Layout geometry generally constitutes a distinctive, robust, and computationally inexpensive cue that can be used in place finding (Cheng 1986; Gallistel 1990; Spelke et al. 2010; Thinus-Blanc et al. 2010; Tommasi et al. 2012; Hohol 2020). This claim alludes to distal evolutionary origins of sensitivity to layout geometry, which imply that it should be observed in various animal phyla. Therefore, we expected to replicate the observations previously seen in studies on vertebrates (Tommasi et al. 1997; Gray et al. 2004; Tommasi and Thinus-Blanc 2004; Tommasi and Giuliano 2014). Nevertheless, one has to remain aware of the possible variance of mechanisms among the species (Vallortigara 2018). Our hypotheses were further substantiated by previous findings that insects exhibit sensitivity to object symmetry (Giurfa et al. 1996; Møller and Sorci 1998; Rodríguez et al. 2004; White and Kemp 2020), and they can tactilely recognize previously seen objects while in the dark (Solvi et al. 2020). Furthermore, insects exhibit good navigational performance in tasks where the spatial layout is geometrically regular (Wystrach and Beugnon 2009; Sovrano et al. 2012; Lee and Vallortigara 2015). Finally, they are capable of swift conceptual learning involving the development of spatial concepts (Giurfa 2013, 2015; Avargues-Weber and Giurfa 2013).
We found that crickets can learn to localize a centrally positioned, inconspicuous cool spot. The additional tests corroborated the inaccessibility of visual cues, and thus supported the effect observed in the main experiment. The learning was significantly more efficient in all the symmetric arenas (circular, square, and triangular) in comparison to the asymmetric quadrilateral one. More specifically, the learning curve estimates were significantly higher in the symmetric arenas than in the asymmetric one, and the latency in finding the center was significantly longer in the latter. This effect was indicated by a higher intercept estimate in the regression model. Nevertheless, in subsequent trials (in all the arenas), the time spent in the center increased and the latency decreased—albeit, in the asymmetric arena, the effect was less pronounced. Furthermore, in all the symmetric arenas, the number of successful approaches was substantially higher, in comparison to the asymmetric quadrilateral one. The data obtained from the spontaneous exploration conditions emphasizes the learning aspect of the observed behavior as the cricket, aside from learning to find the center, had to suppress its thigmotaxis reflex. The spontaneous visits to the center of the arenas were rare in both (heated and unheated) conditions. Aversion to open spaces exhibited by crickets could also account for crickets leaving the cool spot after discovering it. While the crickets’ mode of spontaneous exploration of the arenas differed, those differences do not seem to translate to the results of the learning trials. Furthermore, while the presence of the corners significantly increased the time spent by the crickets in the proximity of the walls, it was not significantly different in the quadrilateral arena. This data seems to exclude the possibility that differences in learning were driven only by the preference for sharp corners. Additionally, overall exploratory activity (as indicated by the traveled distance) does not seem to be related to the complexity of the arenas. Overall, the results corroborate our hypotheses, allowing us to infer that layout symmetry facilitates spatial learning and can be considered a viable cue for successful place finding in insects.
A significant reduction in observed latency indicates that the cricket's capacity to find the center could not be explained entirely in terms of learning to interrupt the random search (or scanning) when the cool spot is reached. If this were so, the time spent on the cool spot would have increased, but the latency would have remained constant (Foucaud et al. 2010). Furthermore, other studies performed using the MWM test revealed that executing heuristic search routines, such as scanning or chaining, could lead to latencies characterizing direct search and related strategies (Wolfer and Lipp 2000; Garthe et al. 2009). Our approach path analysis revealed the employment of both heuristic and direct strategies (Figs. 11, 12), with substantial dominance of a semi-directed strategy (i.e., thigmotactic phase preceded the direct navigation to the target spot) in all the arenas (Fig. 13). Before most of the successful navigation bouts, insects tended to visit the perimeter of the arena and subsequently travel directly to the cool spot (Fig. 12). We consider that the aforementioned thigmotactic phase preceding the travel to the cool spot could be interpreted as an orientation period used to calibrate the memorized allocentric layout model. As the employed experimental paradigm is devoid of visual cues, insects had to rely solely on tactile cues, in contrast to, e.g., rats in MWM, which could instantaneously access visual information about their position. By its distal nature, the visual information provides an overview of a much larger area. This result may be considered as supporting evidence for the memorizing of arenas’ layouts by insects (Gould 1986; Wehner and Menzel 1990; Webb 2019).
Furthermore, as was shown in the third cluster, insects were able to return to the cool spot without performing the extensive thigmotactic orientation phase. Therefore, it seems that aside from allocentric memory of the arena layout, insects were able to retain egocentric memory (this behavior could be comparable to returning to the memorized location using, e.g., path integration vector) of the cool spot’s location and utilize it to navigate back in cases of brief detours from it. Approach paths grouped in clusters 1 and 2 highly resemble non-direct, heuristic search strategies, namely chaining and scanning (Vouros et al. 2018). They consist of repeatable movement patterns, respectively spiral-like and consisting of arch-like detours from the wall, which are executed until the cool spot is reached. Interestingly, those strategies were rarely utilized in the asymmetric arena, and out of the few insects that managed to locate the cool spot, most were using the semi-directed strategy (as defined previously: consisting of orientation phase preceding direct approach) (Graziano et al. 2003).
This indicates that, in principle, it is possible for crickets to successfully navigate in asymmetrical arenas, though for some reason, it is exceedingly more challenging. We speculate that the informational complexity of the arenas may explain one probable cause of this effect. Symmetric arenas are computationally easier to encode (e.g., a circular arena could be described only by its radius; all the walls and angles of a square and triangle arenas are equal) in contrast to the asymmetric quadrilateral arena (in which all walls lengths and angles differ). In contrast, crickets in the wild have to navigate in much more complex environments than simple testing arenas. However, in natural environments, they are constantly provided with information from more than one modality, and some results suggest that multimodal information may facilitate spatial learning (Taevs et al. 2010; Hebets et al. 2014; Buehlmann et al. 2020; Sun et al. 2021). Such possible information processing could be executed by the central complex, as was proposed by Xuelong Sun et al.’s (2021) model. The central complex receives projections from antennal lobes, which are known to process—aside from chemosensory information—the tactile stimuli (Nishino et al. 2005). Hence, we propose that in the face of such limited cues, encoding the asymmetric quadrilateral arena could exceed the working memory capacity of most of the crickets, thus preventing them from successfully orienting in the arena during the thigmotactic phase.
We consider the results obtained in the present study vital for the progress of the debate on insect navigational capabilities. The elucidation of the exact mechanism of center finding and its extent requires further studies, for instance, through testing whether the training in one set of symmetric figures would facilitate the center finding in other similar figures (Tommasi and Thinus-Blanc 2004). Additionally, the reliance of the observed mechanism on tactile cues may be tested, e.g., by removal of the antennae and/or injection of compounds disrupting the function of the mechanoreceptors (e.g., pymetrozine) (Couzin-Fuchs et al. 2015).
Concluding points
-
Crickets are capable of learning to localize a centrally positioned, inconspicuous cool spot.
-
The symmetry of the arena significantly facilitates crickets’ learning to find a cool spot.
-
Crickets used both heuristic and directed strategies of approaching the target, with a dominance of a semi-directed strategy (the thigmotactic phase preceding direct navigation to the target).
-
We hypothesize that the poor performance of crickets in the asymmetrical quadrilateral arena may be explained by the difficulty of encoding its layout with cues from a single modality.
-
We propose that further exploration of observed effects may be followed by testing the spatial learning in other arenas, for example rectangular or rhomboidal ones.
-
The possible involvement of informational inputs from antennal lobes in navigation in the presented task could be studied with experiments involving either removal of the antennae or lesions to the antennal lobes.
Data availability
Raw data are available at the Open Science Framework: https://osf.io/w7db2/. All the further information necessary to replicate the study and its results is available from the corresponding authors upon request.
References
Alary F, Goldstein R, Duquette M et al (2008) Tactile acuity in the blind: a psychophysical study using a two-dimensional angle discrimination task. Exp Brain Res 187:587–594. https://doi.org/10.1007/s00221-008-1327-7
Avargues-Weber A, Giurfa M (2013) Conceptual learning by miniature brains. Proceedings of the Royal Society b: Biological Sciences 280:20131907. https://doi.org/10.1098/rspb.2013.1907
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bivand R, Rundel C (2021) rgeos: Interface to geometry engine - Open source ('GEOS’), https://r-forge.r-project.org/projects/rgeos/. Accessed 10 July 2022
Boguszewski P (2022) BehaView, http://www.pmbogusz.net/?a=behaview. Accessed 10 July 2022
Buehlmann C, Mangan M, Graham P (2020) Multimodal interactions in insect navigation. Anim Cogn 23:1129–1141. https://doi.org/10.1007/s10071-020-01383-2
Butterworth B (2022) Can fish count? What animals reveal about our uniquely mathematical mind, Quercus, London
Cheng K (1986) A purely geometric module in the rat’s spatial representation. Cognition 23:149–178. https://doi.org/10.1016/0010-0277(86)90041-7
Cheng K, Huttenlocher J, Newcombe NS (2013) 25 years of research on the use of geometry in spatial reorientation: a current theoretical perspective. Psychon Bull Rev 20:1033–1054. https://doi.org/10.3758/s13423-013-0416-1
Cheung A, Stürzl W, Zeil J, Cheng K (2008) The information content of panoramic images II: View-based navigation in nonrectangular experimental arenas. J Exp Psychol Anim Behav Process 34:15–30. https://doi.org/10.1037/0097-7403.34.1.15
Collett TS, Collett M (2002) Memory use in insect visual navigation. Nat Rev Neurosci 3:542–552. https://doi.org/10.1038/nrn872
Collett TS, Rees JA (1997) View-based navigation in hymenoptera: Multiple strategies of landmark guidance in the approach to a feeder. J Comp Physiol A 181:47–58. https://doi.org/10.1007/s003590050092
Collett M, Chittka L, Collett TS (2013) Spatial memory in insect navigation. Curr Biol 23:R789–R800. https://doi.org/10.1016/j.cub.2013.07.020
Couzin-Fuchs E, Gal O, Holmes P, Ayali A (2015) Differential control of temporal and spatial aspects of cockroach leg coordination. J Insect Physiol 79:96–104. https://doi.org/10.1016/j.jinsphys.2015.06.007
Cymborowski B (1973) Control of the circadian rhythm of locomotor activity in the house cricket. J Insect Physiol 19:1423–1440. https://doi.org/10.1016/0022-1910(73)90173-X
Dehaene S (2011) The number sense, Revised. Oxford University Press, Oxford
Doria MD, Morand-Ferron J, Bertram SM (2019) Spatial cognitive performance is linked to thigmotaxis in field crickets. Anim Behav 150:15–25. https://doi.org/10.1016/j.anbehav.2019.01.022
Duval A (2019) The representation selection problem: Why we should favor the geometric-module framework of spatial reorientation over the view-matching framework. Cognition 192:103985–104004. https://doi.org/10.1016/j.cognition.2019.05.022
Foucaud J, Burns JG, Mery F (2010) Use of spatial information and search strategies in a Water Maze Analog in Drosophila melanogaster. PLoS ONE. https://doi.org/10.1371/journal.pone.0015231
Gallistel C (1990) The organization of learning. The MIT Press, Cambridge, MA
Garthe A, Behr J, Kempermann G (2009) Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. https://doi.org/10.1371/journal.pone.0005464
Giurfa M (2013) Cognition with few neurons: higher-order learning in insects. Trends Neurosci 36:285–294. https://doi.org/10.1016/j.tins.2012.12.011
Giurfa M (2015) Learning and cognition in insects. Wiley Interdiscip Rev Cogn Sci 6:383–395. https://doi.org/10.1002/wcs.1348
Giurfa M, Eichmann B, Menzel R (1996) Symmetry perception in an insect. Nature 382:458–461. https://doi.org/10.1038/382458a0
Giurfa M, Zhang S, Jenett A et al (2001) The concepts of ‘sameness’ and ‘difference’ in an insect. Nature 410:930–933. https://doi.org/10.1038/35073582
Górska-Andrzejak J, Wojtusiak J (2003) A comparative study of the level of locomotor activity throughout postembryonic development of two cricket species: Acheta domesticus L. and Gryllus bimaculatus de Geer (Ensifera: Gryllidae). J Insect Behav 16:845–857. https://doi.org/10.1023/B:JOIR.0000018324.48914.0c
Gould JL (1986) The locale map of honey bees: do insects have cognitive maps? Science 232:861–863. https://doi.org/10.1126/science.232.4752.861
Graham P, Cheng K (2009) Ants use the panoramic skyline as a visual cue during navigation. Curr Biol 19:R935–R937. https://doi.org/10.1016/j.cub.2009.08.015
Gray ER, Spetch ML, Kelly DM, Nguyen A (2004) Searching in the center: pigeons (Columba livid) encode relative distance from walls of an enclosure. J Comp Psychol 118:113–117. https://doi.org/10.1037/0735-7036.118.1.113
Graziano A, Petrosini L, Bartoletti A (2003) Automatic recognition of explorative strategies in the Morris water maze. J Neurosci Methods 130:33–44. https://doi.org/10.1016/S0165-0270(03)00187-0
Hebets EA, Aceves-Aparicio A, Aguilar-Argüello S et al (2014) Multimodal sensory reliance in the nocturnal homing of the amblypygid Phrynus pseudoparvulus (Class Arachnida, Order Amblypygi)? Behav Proc 108:123–130. https://doi.org/10.1016/j.beproc.2014.09.014
Herzmann D, Labhart T (1989) Spectral sensitivity and absolute threshold of polarization vision in crickets: a behavioral study. J Comp Physiol A 165:315–319. https://doi.org/10.1007/BF00619350
Hohol M (2020) Foundations of geometric cognition. Routledge, London-New York. https://doi.org/10.4324/9780429056291
Hohol M, Baran B, Krzyżowski M, Francikowski J (2017) Does spatial navigation have a blind-spot? Visiocentrism is not enough to explain the navigational behavior comprehensively. Front Behav Neurosci. https://doi.org/10.3389/fnbeh.2017.00154
Izard V, Sann C, Spelke ES, Streri A (2009) Newborn infants perceive abstract numbers. Proc Natl Acad Sci U S A 106:10382–10385. https://doi.org/10.1073/pnas.0812142106
Judd SPD, Collett TS (1998) Multiple stored views and landmark guidance in ants. Nature 392:710–714. https://doi.org/10.1038/33681
Kamil AC, Jones JE (1997) The seed-storing corvid Clark’s nutcracker learns geometric relationships among landmarks. Nature 390:276–279. https://doi.org/10.1038/36840
Kamil AC, Jones JE (2000) Geometric rule learning by Clark’s nutcrackers (Nucifraga columbiana). J Exp Psychol Anim Behav Process 26:439–453. https://doi.org/10.1037/0097-7403.26.4.439
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmertest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.1837/jss.v082.i13
Lachenicht MW, Clusella-Trullas S, Boardman L et al (2010) Effects of acclimation temperature on thermal tolerance, locomotion performance and respiratory metabolism in Acheta domesticus L. (Orthoptera: Gryllidae). J Insect Physiol 56:822–830. https://doi.org/10.1016/j.jinsphys.2010.02.010
Lee SA, Vallortigara G (2015) Bumblebees spontaneously map location of conspecific using geometry and features. Learn Motiv 50:32–38. https://doi.org/10.1016/j.lmot.2014.10.004
Lent DD, Graham P, Collett TS (2013) Visual scene perception in navigating wood ants. Curr Biol 23:684–690. https://doi.org/10.1016/j.cub.2013.03.016
Lochmatter T, Roduit P, Cianci C, Correll N, Jacot J, Martinoli A (2008) SwisTrack: A flexible open source trackingsoftware for multi-agent systems. EEE/RSJ International Conference on Intelligent Robots and Systems 4004–4010 https://doi.org/10.1109/IROS.2008.4650937
Marlair C, Pierret E, Crollen V (2021) Geometry intuitions without vision? A study in blind children and adults. Cognition 216:104861. https://doi.org/10.1016/j.cognition.2021.104861
Mathis A, Mamidanna P, Cury KM et al (2018) DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21:1281–1289. https://doi.org/10.1038/s41593-018-0209-y
McLean DJ, Skowron Volponi MA (2018) trajr: An R package for characterisation of animal trajectories. Ethology 124:440–448. https://doi.org/10.1111/eth.12739
Mizunami M, Weibrecht JM, Strausfeld NJ (1998) Mushroom bodies of the cockroach: their participation in place memory. J Comp Neurol 402:520–537. https://doi.org/10.1002/(SICI)1096-9861(19981228)402:4%3c520::AID-CNE6%3e3.0.CO;2-K
Möller R (2001) Do insects use templates or parameters for landmark navigation? J Theor Biol 210:33–45. https://doi.org/10.1006/jtbi.2001.2295
Møller AP, Sorci G (1998) Insect preference for symmetrical artificial flowers. Oecologia 114:37–42. https://doi.org/10.1007/s004420050417
Morris RGM (1981) Spatial localization does not require the presence of local cues. Learn Motiv 12:239–260. https://doi.org/10.1016/0023-9690(81)90020-5
Müller M (2007) Dynamic Time Warping. Information Retrieval for Music and Motion. Springer, Berlin, Heidelberg, pp 69–84
Nardi D, Singer KJ, Price KM et al (2021) Navigating without vision: spontaneous use of terrain slant in outdoor place learning. Spat Cogn Comput 21:235–255. https://doi.org/10.1080/13875868.2021.1916504
Nieder A (2017) Magnitude codes for cross-modal working memory in the primate frontal association cortex. Front Neurosci 11:202. https://doi.org/10.3389/fnins.2017.00202
Nishino H, Nishikawa M, Yokohari F, Mizunami M (2005) Dual, multilayered somatosensory maps formed by antennal tactile and contact chemosensory afferents in an insect brain. J Compara Neurol 493:291–308. https://doi.org/10.1002/cne.20757
Ofstad TA, Zuker CS, Reiser MB (2011) Visual place learning in Drosophila melanogaster. Nature 474:204–209. https://doi.org/10.1038/nature10131
Potì P, Kanngiesser P, Saporiti M et al (2010) Searching in the Middle-Capuchins’ (Cebus apella) and Bonobos’ (Pan paniscus) behavior during a spatial search task. J Exp Psychol Anim Behav Process 36:92–109. https://doi.org/10.1037/a0015970
Poulet JFA, Hedwig B (2005) Auditory orientation in crickets: pattern recognition controls reactive steering. Proc Natl Acad Sci 102:15665–15669. https://doi.org/10.1073/pnas.0505282102
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reeve RE, Webb B (2003) New neural circuits for robot phonotaxis. Philos Trans Royal Soc: Math, Phys Eng Sci 361:2245–2266. https://doi.org/10.1098/rsta.2003.1274
Rodríguez I, Gumbert A, de Ibarra NH et al (2004) Symmetry is in the eye of the “beeholder”: Innate preference for bilateral symmetry in flower-naïve bumblebees. Naturwissenschaften 91:374–377. https://doi.org/10.1007/s00114-004-0537-5
Sardá-Espinosa A (2019) Time-series clustering in R using the dtwclust package. R J 11:22–43. https://doi.org/10.32614/RJ-2019-023
Sguanci S, Ceccolini F, Berti R (2010) Non visual discrimination of shapes in the blind cave cyprinid Phreatichthys andruzzii Vinciguerra 1924. Ethol Ecol Evol 22:353–358. https://doi.org/10.1080/03949370.2010.510038
Solvi C, Al-Khudhairy SG, Chittka L (2020) Bumble bees display cross-modal object recognition between visual and tactile senses. Science 367:910–912. https://doi.org/10.1126/science.aay8064
Sovrano VA, Rigosi E, Vallortigara G (2012) Spatial reorientation by geometry in bumblebees. PLoS ONE 7:e37449. https://doi.org/10.1371/journal.pone.0037449
Sovrano VA, Potrich D, Foà A, Bertolucci C (2018) Extra-visual systems in the spatial reorientation of cavefish. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-36167-9
Sovrano VA, Baratti G, Potrich D, Bertolucci C (2020) The geometry as an eyed fish feels it in spontaneous and rewarded spatial reorientation tasks. Sci Rep 10:1–14. https://doi.org/10.1038/s41598-020-64690-1
Spelke ES, Lee SA, Izard V (2010) Beyond core knowledge: natural geometry. Cogn Sci 34:863–884. https://doi.org/10.1111/j.1551-6709.2010.01110.x
Stürzl W, Cheung A, Cheng K, Zeil J (2008) The information content of panoramic images I: the rotational errors and the similarity of views in rectangular experimental arenas. J Exp Psychol Anim Behav Process 34:1–14. https://doi.org/10.1037/0097-7403.34.1.1
Sun X, Yue S, Mangan M (2021) How the insect central complex could coordinate multimodal navigation. Elife 10:1–21. https://doi.org/10.7554/eLife.73077
Sutton JE, Newcombe NS (2014) The hippocampus is not a geometric module: processing environment geometry during reorientation. Front Hum Neurosci 8:244. https://doi.org/10.3389/fnhum.2014.00596
Sutton JE, Olthof A, Roberts WA (2000) Landmark use by squirrel monkeys (Saimiri sciureus). Anim Learn Behav 28:28–42. https://doi.org/10.3758/BF03199770
Taevs M, Dahmani L, Zatorre RJ, Bohbot VD (2010) Semantic elaboration in auditory and visual spatial memory. Front Psychol 1:1–10. https://doi.org/10.3389/fpsyg.2010.00228
Thinus-Blanc C, Chabanne V, Tommasi L et al (2010) The encoding of geometry in various vertebrate spacies. In: Dolins FL, Mitchel RW (eds) Spatial Cognition, Spatial Perception. Cambridge University Press, Cambridge, pp 99–116
Tommasi L, Giuliano A (2014) Evidence of a relational spatial strategy in learning the centre of enclosures in human children (Homo sapiens). Behav Proc 106:172–179. https://doi.org/10.1016/j.beproc.2014.06.004
Tommasi L, Thinus-Blanc C (2004) Generalization in place learning and geometry knowledge in rats. Learn Mem 11:153–161. https://doi.org/10.1101/lm.60904
Tommasi L, Vallortigara G, Zanforlin M (1997) Young chickens learn to localize the centre of a spatial environment. J Comp Physiol A 180:567–572. https://doi.org/10.1007/s003590050073
Tommasi L, Chiandetti C, Pecchia T et al (2012) From natural geometry to spatial cognition. Neurosci Biobehav Rev 36:799–824. https://doi.org/10.1016/j.neubiorev.2011.12.007
Vallortigara G (2018) Comparative cognition of number and space: the case of geometry and of the mental number line. Philos Trans Royal Soc b: Biolog Sci 373:20170120–20170128. https://doi.org/10.1098/rstb.2017.0120
Vouros A, Gehring T, v., Szydlowska K, et al (2018) A generalised framework for detailed classification of swimming paths inside the Morris Water Maze. Sci Rep 8:1–15. https://doi.org/10.1038/s41598-018-33456-1
Webb B (2019) The internal maps of insects. J Exp Biol. https://doi.org/10.1242/jeb.188094
Webb B, Wystrach A (2016) Neural mechanisms of insect navigation. Curr Opin Insect Sci 15:27–39. https://doi.org/10.1016/j.cois.2016.02.011
Wehner R, Menzel R (1990) Do insects have cognitive maps? Annu Rev Neurosci 13:403–414. https://doi.org/10.1146/annurev.neuro.13.1.403
Wehner R, Michel B, Antonsen P (1996) Visual navigation in insects: coupling of egocentric and geocentric information. J Exp Biol 199:129–140
Wessnitzer J, Mangan M, Webb B (2008) Place memory in crickets. Proc Royal Soc b: Biolog Sci 275:915–921. https://doi.org/10.1098/rspb.2007.1647
White TE, Kemp DJ (2020) Spider lures exploit insect preferences for floral colour and symmetry. Evol Ecol 34:543–553. https://doi.org/10.1007/s10682-020-10047-z
Wolfer DP, Lipp H-P (2000) Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Exp Physiol 85:627–634. https://doi.org/10.1111/j.1469-445X.2000.02095.x
Wystrach A, Beugnon G (2009) Ants learn geometry and features. Curr Biol 19:61–66. https://doi.org/10.1016/j.cub.2008.11.054
Wystrach A, Graham P (2012a) What can we learn from studies of insect navigation? Anim Behav 84:13–20. https://doi.org/10.1016/j.anbehav.2012.04.017
Wystrach A, Graham P (2012b) View-based matching can be more than image matching: the importance of considering an animal’s perspective. Iperception 3:547–549. https://doi.org/10.1068/i0542ic
Wystrach A, Cheng K, Sosa S, Beugnon G (2011) Geometry, features, and panoramic views: ants in rectangular arenas. J Exp Psychol Anim Behav Process 37:420–435. https://doi.org/10.1037/a0023886
Yamawaki Y, Ishibashi W (2014) Antennal pointing at a looming object in the cricket Acheta domesticus. J Insect Physiol 60:80–91. https://doi.org/10.1016/j.jinsphys.2013.11.006
Zahedi MS, Zeil J (2018) Fractal dimension and the navigational information provided by natural scenes. PLoS ONE 13:e0196227. https://doi.org/10.1371/journal.pone.0196227
Acknowledgements
This study was funded by the Polish Ministry of Science and Higher Education (the Diamond Grant program, id: DI2015025445, PI: Bartosz Baran). MH research has been supported by a grant from the Priority Research Area FutureSoc under the Strategic Programme Excellence Initiative at Jagiellonian University. The authors would like to thank Barbara Webb and Antoine Wystrach for their comments on experimental design and preliminary results, Véronique Izard, Nora Newcombe and Lars Chittka for their helpful remarks regarding the analyses and conceptual framework, and Rafał Czajkowski and Jan Manuel Rodriguez Parkitna for reading and commenting on the very early drafts. Moreover, we would like to thank Ken Cheng and the anonymous reviewers who provided insightful comments on the previous version of this manuscript and encouraged us to include auxiliary control conditions as well as the approach trajectories analysis. We also thank Sylwia Butkiewicz for reading the final draft.
Author information
Authors and Affiliations
Contributions
BB and MH: wrote the manuscript. All authors commented on the manuscript. BB, MK, JF, and MH: conceived, designed, and performed the experiment. BB, ZR, and JF: analyzed the data and prepared the figures and tables. All authors substantially contributed to the interpretation of the data. All authors read and approved the submitted manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Bartosz Baran declares that he has no conflict of interest. Michał Krzyżowski declares that he has no conflict of interest. Zoltán Rádai declares that he has no conflict of interest. Jacek Francikowski declares that he has no conflict of interest. Mateusz Hohol declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. The methods and procedures conformed to guidelines of the Faculty of Natural Sciences of the University of Silesia in Katowice for testing invertebrate animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baran, B., Krzyżowski, M., Rádai, Z. et al. Geometry-based navigation in the dark: layout symmetry facilitates spatial learning in the house cricket, Acheta domesticus, in the absence of visual cues. Anim Cogn 26, 755–770 (2023). https://doi.org/10.1007/s10071-022-01712-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-022-01712-7