Publication Information
ISSN 2692-1529
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Prediction of the distribution of shrimp species found in southern Benin through the lake Nokoué-Ocean complex
Sèlomè Wilfried Sintondji1, 3*, Amoussou Sylvain Gozingan2, 3, Zacharie Sohou1, 3, Matthieu Taymans4, Katrijn Baetens4, Geneviève Lacroix4 & Emile Didier Fiogbé1
1Laboratoire de Recherche sur les Zones Humides, Faculté des Sciences et Techniques, Université d’Abomey-Calavi, 01 BP 526 Cotonou, République du Bénin
2 ICMPA - UNESCO CHAIR, Faculté des Sciences et Techniques, Université d’Abomey-Calavi
3Institut de Recherches Halieutiques et Océanologiques du Benin (IRHOB), 03 BP 1665 Cotonou, République du Bénin
4Royal Belgian Institute of Natural Sciences, Operational Directorate Natural Environment, 29 Rue Vautier, 1000 Brussels, Belgium
Received Date: May 11, 2022; Accepted Date: May 21, 2022; Published Date: May 27, 2022;
*Corresponding author : Sèlomè Wilfried Sintondji, Laboratoire de Recherche sur les Zones Humides, Faculté des Sciences et Techniques, Université d’Abomey-Calavi, 01 BP 526 Cotonou, République du Bénin.
Email : sintondjiwilfried@gmail.com
Citation: Sintondji S W, Gozingan A S, Sohou Z, Taymans M, Baetens K, et al. (2022) Prediction of the distribution of shrimp species found in southern Benin through the lake Nokoué-Ocean complex. Jr Aqua Mar Bio Eco: JAMBE-116.
DOI: 10.37722/JAMBE.2022201
Abstract
The export of shrimps to the European Union was one of the mainstays of the Beninese economy. It is an income-generating activity for the populations living along Lake Nokoué. The lack of a fisheries management strategy has caused a drastic decline in shrimp’s production in Lake Nokoué since 2003. To remedy this problem, it is necessary to hypothesize on their spatial-temporal dynamics in the Lake Nokoué-Cotonou channel complex. This is investigated by combining a literature review on their life cycles and habitat suitability with spatial-temporal evolution of salinity obtained from in-situ observations. The literature review reported four (04) potential families of shrimp species whose part of their life cycle is common to the ecology of Lake Nokoué: Penaeidae (Penaeus); Palaemonidae (Macrobrachium); Atyidae (Atya) and Desmocarididae (Desmocaris). The overall results in relation to the life cycle of the shrimp species indicate that adults of the Macrobrachium, Atya species have a favorable environment in Lake Nokoué from August to November and their larvae must remain in the lake from December to June to ensure their survival. The species of the genus Desmocaris, which carry out their entire life cycle in fresh water, can stay in Lake Nokoué from August to November. The species of the genus Penaeus have a favorable environment in Lake Nokoué from December to June but their larvae can only survive in the sea. Species of the genus Caridina (freshwater) and Palaemon (estuary and marine) belonging to the family Palaemonidae can however be found in Lake Nokoué because of their wide distribution on the West African coast although they are not reported in Benin. This research makes it possible to predict the presence or absence of shrimp genera in a lake system, based on their life cycle. Based on the results obtained, we suggest a ban on fishing in Lake Nokoué between December and February and a ban on shrimp fishing in the Cotonou canal for a good management of the shrimp stock in Benin.
Keywords: Caridea; Crustacea; Habitat Map; Habitat Suitability Study; Migration Season; Penaeoidea; Salinity
Introduction
The shrimps industry has an important place in the world because of its high commercial value. The shrimps occupies on the market 70.5 % of the world production of crustaceans and 15.3% of the world halieutic production. These crustaceans dominated by shrimps are marketed in the world and occupy the second rank after fish, representing 21.7% of the total sales of the major fishery groups [1]. It now represents 84.1% of the live weight of all crustaceans in the world [2]. In Benin, inland fishing accounts for 80% of the national fishery and is one of the main income-generating activities for the populations living along the water bodies and rivers [3]. The main source of fishing is Lake Nokoué, which until 2003 provided more than 80% of the total fisheries production (29,734 tons) of the water bodies of the three departments of southern Benin [4]. The share of the shrimp’s fishery from Lake Nokoué is estimated at two-thirds of the total shrimps supply, the share from Lake Ahémé and the Porto Novo lagoon combined was one-sixth, and the remaining one-sixth came from other small lakes in Benin [5]. The literature has reported in Benin the presence of four (04) families whose part of their life cycle is common to the environment of Lake Nokoué. These are potentially: Penaeidae (Penaeus); Palaemonidae (Macrobrachium); Atyidae (Atya) and Desmocarididae (Desmocaris) [6-9]. The main shrimps species caught in lake Nokoué is Penaeus notialis followed by Penaeus monodon, Penaeus kerathurus and some freshwater shrimps species such as those of the genus Macrobrachium [4, 8, 10]. The species Penaeus notialis accounted for more than 97% of the total shrimp’s production in the country. It is also caught in other West African countries such as Ivory Coast, Senegal, Madagascar, Cameroon and Nigeria [10, 11]. On the economic level, shrimp fishing in Benin has appeared in several studies among the six best sectors, presenting assets for economic growth [12]. From the start of shrimp exports around 1993, until 2002 when shrimp fishing reached its peak, shrimp fishery production was about 7,000 tons for a value of 3.2 billion CFA francs, or about 49 million euros [13, 14]. Shrimps had become the second most important export product after cotton by 2002 [10]. The shrimp sector provided income to 45,000 fishermen, 18,500 women intermediate traders, 150 collectors recognized by exporting companies, 50 permanent employees and 1,200 seasonal employees (mainly women) of exporting companies: DIAX, CRUSTAMER, SOBEP and FSG [10, 15, 17]. In total, the shrimp sector has created nearly 65,000 jobs [17]. When dependents are included, then this sector contributed to the livelihoods of about 250,000 people in Benin or 4% of the Beninese population [10]. Shrimp exports declined rapidly since 2003 due to poor management and lack of regulation of exporting companies (reasons highlighted by the Food and Veterinary Office/EU in 2002). Thus, Benin launched a self-suspension of shrimp exports to the European Union in June 2003. Despite the lifting of this self-suspension in 2005, the shrimp export sector is struggling to resume. Indeed, statistical data clearly show that the quantity of shrimps exported from Benin has dropped from 630 tons in 2002 to 1.5 tons in 2009 [10]. Recent investigations have shown a lack of monitoring of shrimp exports from Benin with strong pressure from local fishermen on Lake Nokoué throughout the year [13, 14, 18]. It is therefore imperative to improve our knowledge on the seasonal distribution of shrimps between the tributaries (So; Ouémé) of Lake Nokoué and the sea for action planning of good management of the shrimp industry in Benin. Located in southeastern Benin, the lake Nokoué, which is the subject of this study, represents the largest area (150 km2) of brackish water in Benin [19]. The lake is in direct communication with the sea through the channel and has two main contributary rivers (Ouémé River and the Sô River), this results in a circulation whose direction alternates with the seasons. The exchange of water between Lake Nokoué and the sea gives raise to significant variations in certain parameters, in particular the salinity of the lake [20, 21]. The seasonal variation in the salinity of Lake Nokoué alternately confers a favourable environment for Penaeus (saltwater shrimp) and certain Caridean freshwater shrimp [22]. The main scientific question we are trying to solve in this study is: What should be the potential spatio-temporal distribution of these different shrimp species in Lake Nokoué and the Cotonou channel? To answer this question, we first present a review of the literature on the shrimp species found in southern Benin and their life cycle in the natural environment. Secondly, we will evaluate their potential spatio-temporal distribution in the Lake Nokoué-Cotonou channel complex, by comparing the evolution of the salinity of this complex with their affinity to live in a more or less saline environment. This work aims to complete the information related to the distribution of shrimp found in southern Benin, in order to propose avenues for their management for the Lake Nokoué-Ocean system.
Material and methods
Study area
The study setting in this work is the entire Lake Nokoué-Ocean system (Figure 1). Located in south-eastern Benin between 6° 22′ N and 6° 30′ N and 2° 20′ E to 2° 35′ E, the Lake Nokoué covers an area of 150 km2. This lake is located in a sub-equatorial climate characterized by a long rainy season concentrated between mid-March and mid-July, a short dry season observed between mid-July and mid-September, a short rainy season between mid-September and mid-November, and long dry season between mid-November and mid-March [23]. The average annual water temperature is 27-29 °C and the average annual rainfall is 900-1100 mm [19, 24]. Lake Nokoué is mainly fed with freshwater by tributaries (Ouémé River and Sô River) and it is connected to the brackish ecosystem of Porto-Novo lagoon via the “Totchè” canal to the Atlantic Ocean, via the artificial channel that is the Cotonou channel [25]. The Cotonou channel contributes mainly to the hydrological and environmental fluctuations of the lake. The main tributaries of Lake Nokoué are:
- The Ouémé, with a catchment area of 46,500 km2 and a length of 523 km, crosses the country from north to south. In terms of fresh water supply, it is largely influenced by the rainfall of its upper basin (Upper Ouémé);
- The Sô, with a catchment area of 1,000 km2 and a length of 70 km, is connected to the Ouémé River in high water and maintains a good level of flow in the dry season;
- The Cotonou channel is 4.5 km long, 300 m wide and between 5 and 10 m deep. It is the sea water tributary of Lake Nokoué.
Review of the literature
The objectives of the literature review were:
- To list the shrimp species that are present in the southern Benin region and in Lake Nokoué.
- To synthesize the available knowledge on the life cycle of these different
- To evaluate the influence of chemical and physical parameters such as salinity, hydrology and bathymetry on the distribution of shrimp throughout the Lake Nokoué - Ocean complex.
Information related to shrimp species life cycles, breeding seasons, migrations and affinities were obtained from the databases https://scholar.google.com/; http://www.ask.com; http://www.freefullpdf.com/; http://www.aginternetwork.org; with the combination of the following keywords: Caridea, Penaeoidea, Penaeidae, Palaemonoidea, Atyidae, Macrobrachium, Desmocaris, Atya, Penaeus, migration, reproduction, classification, distribution, ecology, shrimp, fresh, water, salt, cycle, annual, salinity, biology, West, African, coast, Benin. In addition, reports, dissertations and theses were also consulted in the libraries of the Ministry of Agriculture of the Universities and the Directorate of Fisheries of Benin and, the World Register of Marine Species Database (WoRMS 2022, http://marinespecies.org/) was used to update the data related to the classification of shrimp species. A total of 105 theses, 375 scientific articles and 80 technical reports were consulted, whose only 12, 44 and 20 respectively were used. The choice to use them or not was guided by the relevance of the documents that address the topic.
In-situ data and analysis
Salinity and bathymetry data of the Lake Nokoué - Cotonou channel complex were obtained during monthly campaigns carried out at 54 sampling stations (Figure 1) by the “Institut de Recherche Halieutiques et Océanologiques du Bénin” (IRHOB) in collaboration with the “Institut de Recherches et de Développement” (IRD) between November 2017 and August 2018 (http://nodc-benin.odinafrica.org/nous-joindre.html). Vertical profiles were conducted each month to determine depth and salinity using a CTD (Conductivity-Temperature-Depth) probe at each of the 54 stations using a motorized boat. A GPS was used to acquire the geographic coordinates of all sampling stations. For the analysis, monthly surface and bottom salinity data have been interpolated over a 1 km x 1 km grid. Monthly depth data were averaged to obtain the average depth of each station, and then interpolated on the same grid. The Matlab software was used to produce the salinity and bathymetry maps.
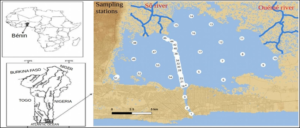
Figure 1: Map of the area of interest and position of the sampling stations on Lake Nokoué and in the Cotonou channel.
Results
Shrimp species present in southern Benin and their life cycle|
Penaeidae
The Penaeidae of the genus Penaeus adopt an anadromous migration linked to their reproductive cycle (Figure 2, left). On the other hand, others carry out their complete cycle at sea [26]. Two groups of species of Penaeidae are reported from Benin: deepwater shrimp: Parapenaeus longirostris and coastal shrimp: Penaeus notialis, Penaeus kerathurus Penaeus monodon, Holthuispenaeopsis atlantica, Metapenaeopsis miersi [7, 8, 9, 27, 28].
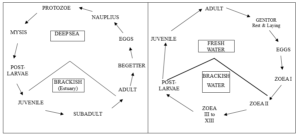
Figure 2: Modified life history diagram of Penaeus (left) [7, 26, 29] and diagram of most freshwater shrimp such as the genus Macrobrachium and Atya (right) [22, 30, 31].
Palaemonidae
Belonging to the superfamily Palaemonoidea, the Palaemonidae Rafinesque, 1815 represents one of the few groups of decapods that have colonized estuaries and rivers in subtropical and tropical regions. Some species of the family Palaemonidae perform a catadromous migration (Figure 2, right) [22]. Others perform an anadromous migration, however some species of the same family as Palaemonidae make their complete life cycle in the sea and others entirely in fresh water. Moreover, the genus Macrobrachium is the most diverse of the family Palaemonidae [32, 33] with currently 256 species described worldwide. In West Africa, there are eleven (11) described species: These are M. chevalieri Roux, 1935; M. dux Lenz, 1910); M. felicinum; M. macrobrachion; M. raridens, M. rosenbergii; M. sollaudii; M. thysi; M. vollenhovenii; M. equidens and M. zariquieyi [9, 34-36]. Its distribution is pantropical, covering the lowlands of Africa, Asia, Oceania, North, Central and South America. Seven species have been reported from Beninese waters: M. dux Lenz, 1910; M. felicinum; M. vollenhovenii; M. macrobrachion; M. raridens; M. sp1 and M. sp2 [6, 9, 37]. Other species of the family Palaemonidae such as Palaemon maculatus have also been reported along the West African coast but not specifically in Benin [9, 38].
Atyidae
Belonging to the superfamily Atyoidea, the Atyidae now includes 46 genera. The genus Atya is characterized by a catadromous life cycle similar to that carried out by most shrimp of the genus Macrobrachium (Figure 2, right) [39, 40]. The genus Atya has fourteen described species, four of which inhabit West African freshwaters: A. africana; A. gabonensis; A. intermedia and A. scabra [35, 41]. Two of them have been reported in Beninese Rivers: A. africana, A. gabonensis [6]. The genus Caridina is represented today in the world with 332 species (http://marinespecies.org/). The genus Caridina could be found in Lake Nokoué, particularly the species C. togoensis which is more widespread in Africa but not specifically in Benin [42].
Desmocarididae
The Desmocarididae family is characterized by a life cycle exclusively in freshwater, i.e. in an environment favourable to the plant Eichhornia crassipes. According to [33], the Desmocarididae are found in freshwater. This family contains only the genus Desmocaris with currently two species (D. bislineata and D. trispinosa) including one found in Benin, which is D. trispinosa [6].
Life cycle of each shrimp species found in southern Benin
Table 1 presents a general summary of the life cycle of shrimp species found in southern Benin. This review was made on the basis of available information along the West African coast from Sierra Leone to Angola. 2011) Metapenaeopsis miersi Palaemonidae _ _ _ Atya africana _ _ _
Species
Areas and periods of reproduction
Larval requirement
Affinity of juveniles
Adult Affinity
Preferred depth
Penaeidae
Penaeus monodon
In the sea; continuous reproduction with a peak between July and March [43]
Sea water [7, 44]
Brackish water [7]
Sea water [7]
Can exceed 30m [7]
Penaeus kerathurus
In the sea; continuous reproduction with a peak from May to mid-November Novembre [7]
Sea water [7]
Brackish water [7, 44]
Sea water [7, 44]
5-50m
[7]
Penaeus notialis
In the sea; continuous reproduction with a peak between July and December [45,46,47,48]
Sea water [7, 44]
Brackish water [7]
Sea water [7] (
10 – 75m [7]
Parapenaeus longirostris
Offshore; Continuous breeding with a peak from November-April [7,9]
Sea water [7, 9]
Sea water [7, 9]
Sea water [7, 9]
100 – 400m [7,9]
Holthuispenaeopsis atlantica
Offshore; Almost continuous all year l’année [9,43,50,51]
Sea water [7, 9, 44]
Sea water [7, 9]
Sea water [7, 9]
10 – 40m [7,9]
M. dux; M. felicinum; M. vollenhovenii; M. macrobrachion; M. raridens; M. sp1 and M. sp2
Lake, lagoon, river or estuary; with a peak during the rainy season pluies [22,30,52,53]
Brackish water Eau saumâtre [30, 52,54]
Fresh water [30, 52]
Fresh water [30, 52]
Palaemon maculatus
Offshore; Almost continuous all year [9]
Sea water, [9]
Brackish water or Sea water [9]
Brackish water or Sea water
Coastal water Up to 50 m [9]
Atyidae
Atya gabonensis;
Lake, lagoon, river or estuary; with a peak during the rainy season [39, 40, 50, 55]
Brackish water [39,40,55]
Fresh water [39,50,55]
Fresh water [39, 40, 50, 55]
Desmocarididae
Desmocaris trispinosa
River, lake or other freshwater. [50, 55]
Fresh water [50, 55]
Fresh water [50, 55]
Fresh water [50, 55]
_ _ _
Relationship between the life cycle of shrimps and the geochemical characteristics of Lake Nokoué.
Bathymetry of Lake Nokoué - Cotonou channel
The depth data allowed us to produce a bathymetric map of Lake Nokoué which showed that the relative elevation of the water level varies between 2 m and 3.2 m on average in the centre of the lake. The depth of the lake varies between 1 m and 2 m to the east and west of the lake. In the north, the depth is between 1 m and 1.6 m, whereas it is close to 3 m in the centre of the lake and in the south of Lake Nokoué near the Cotonou channel. The depth in the Cotonou channel varies between 4 m and 6 m on average (Figure 3). In general, Lake Nokoué has a fairly flat bottom with a very shallow depth. Shrimps are benthic already from the juvenile stages [26, 29, 43, 57]. Therefore, the bathymetric characteristics of Lake Nokoué are suitable for a homogeneous distribution of shrimps because the relatively flat bottom should not favour a strong accumulation of organic debris in a deeper zone.
 Figure 3: Bathymetry of Lake Nokoué (a) and the Cotonou channel (b).
Figure 3: Bathymetry of Lake Nokoué (a) and the Cotonou channel (b).
Seasonal evolution of salinity in Lake Nokoué-Cotonou channel
The interpolated data allowed to generate maps of the spatio-temporal distribution of salinity in the basin of the Lake Nokoué-Cotonou channel complex (Figure 4, 5). These maps showed in December the beginning of saline intrusion from the Atlantic Ocean via the Cotonou channel, on both surface (Figure 4) and bottom (Figure 5). This entry of sea water into Lake Nokoué is progressively increasing from the southern part (entrance to the channel) and progressively extending to the northern side of the basin. This saline intrusion continues to reach the western side first, then progressively the northern and eastern sides of the basin, during the month of January, due to the flow of the Sô River and the Ouémé River. The highest salinity level of the lake is observed in April. During this period, the salinity values of the lake are almost oceanic on the surface as well as on the bottom (salinity > 30 PSU). Lake Nokoué is then more subject to tidal currents than to the low flow of the lake's tributaries, notably the Sô and Ouémé rivers (Figure 4, 5). During the rainy season mid-April to mid-August (Figure 7), there is a significant increase in the flow of the rivers flowing into Lake Nokoué, and the surface salinity of the lake and the channel begins to fall from May to reach very low values between July and August. This desalination of the lake occurs more rapidly on the northeast side than on the southwest side of the basin where a portion of the water on the west side and in the channel remains slightly salty in July. Maps of spatial distributions of salinity show that, overall, the bottom of the lake is proportionally saltier than the surface (Figure 5). During the December-January period, the salinity of the lake increases with the exception of the areas located at the mouth of the Sô and Ouémé rivers which continue to have a low flow into the lake, despite the end of the rains (Figure 7).

Figure 4: Seasonal distribution of surface salinity in Lake Nokoué and the Cotonou channel
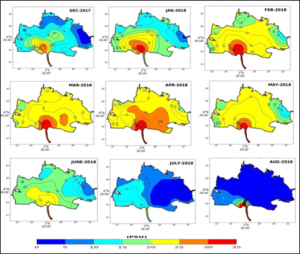
Figure 5: Seasonal distribution of bottom salinity in Lake Nokoué and the Cotonou channel.
Evaluation of the spatio-temporal distribution of different shrimp species according to the bottom salinity in the complex composed of Lake Nokoué and the Cotonou channel
The spatio-temporal distribution of salinity is a determining factor in the spatio-temporal distribution of species in the lake-channel complex. From knowledge about life cycle of the shrimp species, and especially on their affinity for salinity, it appears that the bottom salinity of Lake Nokoué remains favourable for species of the genus Penaeus during the months of December to June (Figure 2 left, 5, and 6). The appearance of juvenile Penaeus (Penaeus notialis, Penaeus kerathurus and Penaeus monodon) and possible species Palaemon maculatus in the channel and Lake Nokoué can already start in December when the salinity of the bottom is higher than 20 PSU in some places. The return to the sea of these species should start in June with the decrease of the salinity of the lake and that of the channel of Cotonou, following the strong entry of fresh water from the Ouémé and Sô rivers. This massive inflow of fresh water into lake Nokoué should lead to the migration of species of the genus Macrobrachium (M. dux; M. felicinum; M. vollenhovenii; M. macrobrachion; M. raridens; M. sp) and those of the genus Atya (A. africana and A. gabonensis) that are entering their reproductive period to lake Nokoué (Figure 2 right, 5, and 6). Adults of the genera Macrobrachium and Atya have an affinity with the fresh water of Lake Nokoué during the months of August to November and begin their migration from Lake Nokoué to the Ouémé River in December (Figure 2 right, 5, and 6). The larvae of the Macrobrachium and Atya species are forced to remain in the brackish waters of Lake Nokoué from December to June, until they reach the post-larval stage to have a chance to survive (Figure 2 right and 5). As for the Desmocaris species (D. trispinosa), which carries out its entire life cycle in the freshwater tributaries of Lake Nokoué and can only pass through Lake Nokoué during the period from August to November when the environment is favourable for the development of the Eichhornia crassipes plant (freshwater). Tables 1 and 2 and Figure 6 summarises the annual migration of shrimp species through the tributaries, Lake Nokoué and the Cotonou channel in Benin. & Subdults Atya africana
Espèces
Stades
Jan
Feb
Mar
Apr
May
Jun
Jul
Aug
Sept
Oct
Nov
Dec
Penaeidae
Penaeus monodon; Penaeus kerathurus; Penaeus notialis
Larvae
+S
+S
+S
+S
+S
+S
+S
+S
+S
+S
+S
+S
Juveniles
+Ln
+Ln
+Ln
+Ln
+Ln
+Ln
±Ln
-Ln
-Ln
-Ln
-Ln
+Ln
Adults
+S
+S
+S
+S
+S
+S
+S
+S
+S
+S
+S
+S
Palaemonidae
M. dux; M. felicinum; M. vollenhovenii; M. macrobrachion; M. raridens; M. sp1 & M. sp2
Larvae
+Ln
+Ln
+Ln
+Ln
+Ln
+Ln
±Ln
-Ln
-Ln
-Ln
-Ln
±Ln
Juveniles & Adults
-Ln
-Ln
-Ln
-Ln
-Ln
-Ln
±Ln
+Ln
+Ln
+Ln
+Ln
±Ln
Atyidae
Atya gabonensis;
Larvae
+Ln
+Ln
+Ln
+Ln
+Ln
+Ln
±Ln
-Ln
-Ln
-Ln
-Ln
±Ln
Juveniles & Adults
-Ln
-Ln
-Ln
-Ln
-Ln
-Ln
±Ln
+Ln
+Ln
+Ln
+Ln
±Ln
Desmocarididae
Desmocaris trispinosa
Larvae
-Ln
-Ln
-Ln
-Ln
-Ln
-Ln
-Ln
±Ln
+Ln
+Ln
±Ln
-Ln
Juveniles & Adults
-Ln
-Ln
-Ln
-Ln
-Ln
-Ln
-Ln
±Ln
+Ln
+Ln
±Ln
-Ln
Note: +S = Present at sea; +Ln = Present in lake Nokoué; -Ln = Absent in lake Nokoué; ±Ln = Present or absent in lake Nokoué

Figure 6: Seasonal distribution of shrimp according to bottom salinity in Lake Nokoué and the Cotonou Channel.(Penaeus)+; (Macrobrachium, Atya, Desmocaris)*
Discussion
The bathymetry of Lake Nokoué is rather shallow and doesn’t show a lot of variation. These results are consistent with those of [58, 59]. The depth of the lake is not expected to influence the distribution of shrimps because, according to the work of [60], it is the nature of the area substrate (dead wood and leaves, invertebrates, and other organic matter) that attracts shrimp and not the depth, even though in deep areas, more debris is present. The shallow depth gives Lake Nokoué the characteristics of a polymictic lake. Thus, Lake Nokoué should regularly undergo mixing. This would prevent any kind of temperature or salinity stratification. However, maps of spatial distributions of salinity in lake Nokoué and the Cotonou channel showed that the bottom of the lake is proportionally more saline over time than the surface of the lake (Figure 4 and 5). These results could be explained by the tidal pressure on Lake Nokoué at the time of saline intrusion. This tidal pressure affects the bottom water compartment before rising to the surface with time [61]. The results of this study allowed us to evaluate the period of shrimp settlement in the lake. Indeed, from December to June, the salinity of lake Nokoué increases, which indicates a favourable environment for Penaeus juveniles: Penaeus monodon, Penaeus kerathurus, Penaeus notialis and possible species Nematopalaemon hastatus, Palaemon elegans and Palaemon maculatus, but also an essential environment for the survival and development of larvae of the Macrobrachium species: M. dux, M. felicinum, M. vollenhovenii, M. macrobrachion, M. raridens, M. sp; and of the genus Atya: Atya gabonensis; Atya africana (Table 1) [7, 22, 40, 54]. These results are confirmed by the results of four months of sampling of Penaeidae conducted by [8] on Lake Nokoué. Juvenile Penaeus are, moreover, abundantly caught in Lake Nokoué from December onwards, as soon as the period of saline intrusion starts [8]. As for the hatching of the eggs of these Penaeus (reproduction), it must therefore take place at sea a few weeks before. On this basis, we estimated that the period of peak reproduction of Penaeus would extend from August to November. Comparing these results with other studies [62-64], it is concluded that the same species of the family Penaeidae could have different reproduction periods in different geographical areas. [65], who studied the biology, reproduction and population dynamics of the deep water shrimp Parapenaeus longirostris at the level of the Algerian west coast (port of Oran and Arzew), showed that the period of strong reproduction observed in Parapenaeus longirostris extends from May to June. This same period has been widely described for P. longirostris in the western Mediterranean, suggesting a minimal reduction mechanism of intra-specific competition [63, 66]. The reproductive period observed for P. longirostris in the waters of the Algerian western coast coincides with other studies carried out in the western Mediterranean (Merbah, 2002) as well as in Italy and Portugal [63, 67]. In contrast, in Senegal, the egg-laying period in P. longirostris is spread over the whole year with two distinct peaks: the most important in winter (February-March) and the second in autumn, October-November [64]. For [68], oviposition in P. longirostris occurs between December and January, with the months of June to August corresponding to the sexual rest period with a resumption of ovarian maturation in September. These differences in the observation of the reproductive period of the same species could be explained by the inequality of climate observed between the West African side and the Algerian west coast. Thus the high temperature highlighted as a factor influencing reproduction by [69], indicates that spawning takes place in P. longirostris in the cold season and in the warm season. In the context of this study, as the Lake Nokoué temperature varies only little (27° C - 29° C), the impact of temperature on the seasonal dynamics of shrimp reproduction has not been taken into account. In the framework of this work, the salinity maps of the basin indicate that freshwater shrimp of the genus Macrobrachium, Atya and Desmocaris have an affinity for Lake Nokoué during the months of August to November. This period of flooding of Lake Nokoué (August to November) marks the end of the rainy seasons in Benin. Since the arrival of freshwater shrimp in Lake Nokoué coincides with their reproduction, it is likely that the rainy season influences or contributes to initiate the gonad maturation period in freshwater shrimp. These results are consistent with those obtained by several researchers [52, 70, 71], who have located the period of egg laying and reproduction during these months based on the study of the ovarian cycle in some freshwater shrimps. According to these authors, the adults Macrobrachium live in the fresh waters of the rivers where the fertilization takes place, especially during the rainy season. Vitellogenesis, according to these authors, is linked to the rainy season and their migration to brackish waters. However, the species belonging to the genus Macrobrachium seem to have two different life cycles because, [72] observed that West African Macrobrachium can be divided into two categories: those with small and numerous eggs and those with large and few eggs. He placed M. dux in the category of species with large and few eggs, as is the case with M. potiuna according to [36, 73] observed that species with small and numerous eggs, such as Macrobrachium vollenhovenii according to [54], have a much wider distribution than those with large and few eggs. [74] Reports many M. dux far inland from the sea in the Democratic Republic of Congo. He also mentions the large size of the eggs in these species. It is difficult to imagine that these shrimp could make migrations of several hundred kilometers to the sea and one must therefore assume that their development is entirely in fresh water. Authors like [75] indicate that the species genera Macrobrachium with large and numerous eggs have a shortened development taking place entirely in fresh water and is met far from the estuaries, while the species with small and numerous eggs have a long larval development taking place in estuarine medium. In addition, species of the genus Caridina, which is a freshwater shrimp with a life cycle similar to that of Macrobrachium, could also be found in Lake Nokoué, in particular the species C. togoensis. But this rather difficult genus continues to create confusion on the systematic level. Indeed, eighteen species of Caridina have been described by [42]. According to the same author, only C. togoensis is more widespread in Africa and could be found in Benin while C. africana has a limited distribution in South Africa. However, [76] recently revealed that all of these descriptions by [42], are nothing more than subspecies of C. africana because of the representativeness of the samples described. We believe in this work that there is a real need for signalling of Caridina in Benin before any suggestions. Rainfall also seems to have a determining role in the beginning and end of saline intrusion through the tributary - Lake Nokoué - ocean complex (Figure 7). Indeed, the beginning of the saline intrusion indicates, according to Météo-Benin data, the end of the short rainy season in Cotonou (December 2017) (Figure 4, 5 and 7). The salinity of Lake Nokoué reaches its peak in April, (period corresponding to the beginning of the long rainy season in Cotonou). We note that during the long rainy season (May 2018), the salinity of Lake Nokoué begins to decrease progressively and practically drops to zero during the short dry season (August 2018).

Figure 7: Rainfall in Cotonou from November 2017 to October 2018 (METEO-Bénin).
Conclusion
By combining information on the life cycle of shrimps found in southern Benin, from the literature, with the spatio-temporal distribution of salinity in the Lake Nokoué-Cotonou channel complex, the evolution of the potential distribution of shrimp species in this complex could be established. The present work has shown that the salinity of Lake Nokoué increases from December to reach a peak in April. The period (December-June) of high salinity in the lake indicates a favourable environment for Penaeus and freshwater shrimp larvae such as Macrobrachium and Atya. Furthermore, the salinity of the water of Lake Nokoué remains very low during the months of August to November, which is the period favourable for the presence of adult freshwater shrimps. Based on the results obtained, we suggest a ban on fishing in Lake Nokoué between December and February. Based on the life cycle of the Penaeus, the beginning of the saline intrusion corresponds to the appearance of juveniles that have not reached sexual maturity. Therefore, fishing between December and February gives less chance to a part of the Penaeus to make their reproductive return to the sea. Secondly, we suggest a ban on shrimp fishing in the Cotonou channel, given that the channel constitutes the only way for the Penaeus to go to and from the lake and the ocean. These two previous recommendations could contribute to a better regulation of the fishery in the Lake Nokoué-channel complex for a sustainable management of the shrimp stock. Complementary studies are underway to evaluate in situ the presence and relative abundance of these species in the Lake Nokoué-Chenal complex and to further investigate their spatio-temporal distribution based on the physical and chemical parameters of the lagoon system. Future research will compare the current faunal richness of the Lake Nokoué-channel complex with that indicated by the literature in southern Benin in general years ago.
Acknowledgement: The authors wish to express their gratitude to Professor Cedric d'Udekem d'Acoz, specialist in crustaceans at RBINS for their advice.
Funding: This work was funded by the CEBioS program (funded by the Belgian Development Cooperation, DGD).
References
- FAO (2016) La situation mondiale des pêches et de l’aquaculture. Contribuer à la sécurité alimentaire et à la nutrition de tous. 224.
- FAO (2020) La situation mondiale des pêches et de l’aquaculture La durabilité en action. Rome. https://doi.org/10.4060/ca9229fr
- Ollabodé N (2019) Analyse économique des chaînes de valeur des crevettes d’eaux douce et saumâtre au sud-Bénin. Thèse de master professionnel, Faculté d’Agronomie, Université de Parakou, Bénin,
- Sohou Z, Houedjissin RC, Ahoyo NR A (2009) La pisciculture au Bénin de la tradition à la modernisation. Bulletin de la Recherche Agronomique du Bénin, Numéro 66 - Décembre
- Allegre, Dupret (2010) Assistance au secteur industriel de la pêche au Bénin pour l’harmonisation du système de production avec la réglementation ID020BEN, Rapport de mission, SFP.
- Kouton M D (2004) Diversité, écologie et exploitation des crevettes d’eau douces dans la basse vallée de l’Ouémé: Cas de la commune d’Adjohoun au Bénin. Mémoire de Thèse d’Ingénieur Agronome, 122
- Sohou Z, Djiman R (2011) Présence de la crevette tigrée, Penaeus monodon Fabricius, 1798 (Crustacea, Penaeidae) dans les eaux maritimes béninoises. Journal de la Recherche Scientifique de l'Université de Lomé, 13:9-17.
- Hinvi LC, Kouhoundji N, Tente B, Agbahungba G, Fiogbe ED, et al. (2013) Caractérisation spatiale des crevettes Penaeidae dans le lac Nokoué à So-Ava (Bénin). Annales des Sciences 390 Agronomiques, 17 (1): 87-101, 2013 Issn 1659-5009.
- Fransen CHJ M (2014) Shrimps and In K.E. Carpenter & N. De Angelis, eds. The living marine resources of the Eastern Central Atlantic. Vol. 1: Introduction, crustaceans, chitons and cephalopods. FAO Species Identification Guide for Fishery Purposes. Rome, FAO. 37-196.
- Houssa R and Verpoorten M (2013) The unintended consequence of an export ban: Evidence from Benin’s shrimp University of Namur. WP 1304 Department of economics Working Papers Series. Centre for Research in the Economics of Development.
- Djirieoulou KC (2017) Peuplement des crevettes des hydrosystèmes de marais et fluvio-lagunaires du sud-est de la Cote d’Ivoire: Diversité, structure, et croissance des Laboratoire d’Hydrobiologie et d’Ecotechnologie des Eaux. Thèse l’Université Félix Houphouët-Boigny Numéro d’ordre 2029/2017.
- Konnon D, Dade A, Montcho H, Gossou D (2009) Etude sur l’industrie agro-alimentaire, facteur d’auto-suffisante alimentaire et de croissance pour l’économie Béninoise. Direction Générale des Affaires Rapport final réalisé par le Centre d’Education à Distance du Bénin (CEDB).
- Adégbola YP, Aquilas F, Samey N, Clohounto J et Soglo YY (2013) Analyse de la compétitivité de la chaîne de valeur ajoutée crevette fraîche et crevette fumée du Bénin, 4th International Conference of the African Association of Agricultural Economists, September 22-25,
- A.E.P (2017) Plan Stratégique de Développement du Secteur Agricole (PSDSA) 2025 et Plan National d’Investissements Agricoles et de Sécurité Alimentaire et Nutritionnelle PNIASAN 2017-2021. Rapport final. Cotonou: MAEP, 135.
- BTC - Belgian Technical Cooperation (2007) Dossier Technique et Financier: Appui au Développement des Filières Halieutiques du Bénin, code DGCD: NN 3003204, 107.
- Le Ry J M, Barry O and Legendre E (2007) Plan de Relance de la Filière Halieutique: Rapport de l’Expert international Court Terme, Projet Appui au Secteur Privée (PASP), République du Bénin, Ministère de l’industrie et du commerce,
- Sachi PS, Bokossa IY, Tchekessi CC, Banon JS, Djogbé A, Bleoussi R (2016) La filière crevette au Bénin, cas des crevettes des eaux saumâtres: Synthèse International Journal of Innovation and Applied Studies, 18:445-457.
- Ollabodé N, Kpadé CP, Montchowui É, Jabi JA (2021) Performance économique des chaînes de valeur des crevettes d’eaux douces au Bénin. Cahiers Agricultures, 30:19.
- Mama D, Deluchat V, Bowen J, Chouti W, Yao B, Gnon B and Baudu M (2011) Caractérisation d'un système lagunaire en zone tropicale: as du lac Nokoué (Bénin). European Journal of Scientific Research, 56:516-528.
- Gnohossou P M (2006) La faune benthique d’une lagune ouest africaine (le lac Nokoué au Benin), diversité, abondance, variations temporelles et spatiales, place dans la chaine Toulouse, France: Institut National Polytechnique de Toulouse.
- Djihouessi MB, Aina MP (2018) A review of hydrodynamics and water quality of lake Nokoué: Current state of knowledge and prospects for further Regional Studies in Marine Science, RSMA 347. https://doi.org/10.1016/j.rsma.2018.01.002
- Griessinger J M, Lacroix D, Godouin P (1991) Elevage de la crevette tropicale d’eau Institut Français de Recherche pour l’Exploitation de Mer; 372.
- Adandedjan D, Ahouansou S, Chikou A, Laleye P, Gourene G (2013) Caractérisation des peuplements de macroinvertébrés benthiques à l’aide de la carte auto-organisatrice (SOM). Comptes Rendus Biologies 336:244-248.
- Amoussou, E (2010) Variabilité pluviométrique et dynamique hydro-sédimentaire du bassin- versant du complexe fluvio-lagunaire Mono-Ahémé-Couffo (Afrique de Couffo). Thèse de Doctorat, France, Université de Bourgogne, CRC, France,
- Frontalini F, Armynot du Châtelet E, Debenay JP, Coccioni R, Bancalà G (2011) Benthic foraminifera in coastal lagoons: distributional patterns and biomonitoring In: New York, Inc, pp 39–72.
- Motoh H (1981) Studies of ficheries biology of the giant tiger prawn, Penaeus monodon in the Philippines. Technical report, n°7, Tigbaun, Iloilo: SEAFDEC Aquaculture Departement,
- Fischer W, Bianchi G et Scott WB, (eds) Fiche FAO (1981) Identification des espèces pour les besoins de la pêche, Atlantique Centre-est; Zone de pêche. 34, 37 (en partie). Canada Fonds de Dépôt. Ottawa, Ministère des pêcheries et Océans Canada, en accord avec l’Organisation des Nations-Unies pour l’Alimentation et l’Agriculture, 1-7.
- Thiaw Fall M & Thiam N (2013) Évaluation par l’approche globale du stock Sénégalais de crevettes profondes, Parapenaeus longirostris (Lucas, 1847). Le Journal des Sciences Halieutique et Aquatique, 7:255-270.
- Rafalimanana T (1990) La pêche maritime traditionnelle [Traditional marine fisheries]. (French). In: "Rapport séminaire national sur les politiques et la planification du developpement des pêches à Madagascar, Antananarivo (Madagascar), 15-19 Oct 1990, Andrianaivojaona."Kasprzyk CZW, Dasylva G. Eds. Ministère de la Production Animale et des Eaux et Forêts, Antananarivo (Madagascar). 224-230.
- Villé J P (1970) Recherches sur la reproduction des Macrobrachium des lagunes I: la fécondité précoce chez les Macrobrachium de Côte d’Ivoire. Annales de l’Université d’Abidjan, série E, 3:253-262.
- Lal MM, Seeto J and Pickering TD (2014) Complete larval development of the Monkey River Prawn Macrobrachium lar (Palaemonidae) using a novel greenwater SpringerPlus, 2014, 3:568.
- Valencia DM & Campos MR (2007) Freshwater prawns of the genus Macrobrachium Bate, 1868 (Crustacea: Decapoda: Palaemonidae) of Colombia. Zootaxa, 1456:1-44.
- De Grave S, Cai Y, Anker A (2008) Global diversity of shrimps (Crustacea: Decapoda: Caridea) in Hydrobiologia, 595:287-293.
- Holthuis LB (1980) FAO species Vol. 1. Shrimps and prawns of the world. An annotated catalogue of species of interest to fisheries. FAO Fisheries Symposium, 1:1-261.
- Monod T (1980) Décapodes. In: Flore et faune aquatiques de l’Afrique sahélo-soudanienne (Durand JR & Lévêque C, eds). ORSTOM, Paris, France, Tome I, 44:369-389.
- Powell CB (1980) The genus Macrobrachium in West Africa: thysi a new large egged species from Ivory Coast (Crustacean, Decapoda, Palaemonidae). Revue de Zoologie Africaine, 94:317-326.
- Agadjihouèdé H (2006) Diversité et exploitation des crevettes d’eau douce dans la lagune de Grand-Popo (Bas Mono). « Ingénieur Agronome » Thesis, Université d’Abomey-calavi.
- Ashelby CW & De Grave S (2009) A new species of Palaemon (Crustacea, Decapoda, Palaemonidae) from West Africa, with a redescription of Palaemon maculatus (Thallwitz, 1892). Zootaxa, 2085:27-44.
- Bauer RT (2011a) Amphidromy and migrations of freshwater shrimps. I. Costs, benefits, evolutionary origins, and an unusual case of amphidromy. In: A. Asakura (ed.). New frontiers in crustacean biology. Proceedings of the Crustacean Society Summer Meeting, Tokyo, 20-24 September 2009, Brill, Leiden, 145-156.
- Herrera-Correal J, Mossolin EC, Wehrtmann IS & Mantelatto FL (2013) Reproductive aspects of the caridean shrimp Atya scabra (Leach, 1815) (Decapoda: Atyidae) in São Sebastião Island, southwestern Atlantic, Brazil. Latin American Journal of Aquatic Research, 41(4): 676-684. https://doi.org/103856/vol41-issue4-fulltext-4
- Page TJ, Cook BD, Von Rintelen T, Von Rintelen K & Hughes J M (2008) Evolutionary relationships of Atyid shrimps imply both ancient Caribbean radiations and common marine dispersals. Journal of the North American Benthological Society, 27 (1): 68-83.
- Richard J & Clark PF (2009) African Caridina (Crustacea: Decapoda: Caridea: Atyidae): redescriptions of C. africana Kingsley, 1882, C. togoensis Hilgendorf, 1893, C. natalensis Bouvier, 1925 and C. roubaudi Bouvier, 1925 with descriptions of 14 new species. Zootaxa, 1995: 1–75.
- Le Reste L (1971) Rythme saisonnier de la reproduction, migration et croissance des post-larves et des jeunes chez la crevette Penaeus indicus H. Milne Edwards de la baie d’ambaro Côte N. 0 De Madagascar: contribution à l’étude d’une baie eutrophique tropicale. Cahiers Office de la Recherche Scientifique et Technique Outre-Mer, série. Océanographie, vol. IX, no 3, 1971: 279-292.
- Domalain G, Rasoanandrasana N and Tiandraza A (2002) Aperçu de l’exploitation et de ses contextes In: La ruée vers l’or rose: Regards croisés sur la pêche crevettière traditionnelle à Madagascar. Marseille:IRD Éditions. https://doi.org/10.4000/books.irdeditions.8434
- Lhomme F (1981) Biologie et dynamique de Penaeus (Farfante Penaeus) notialis Perez farfante, 1967 au Sénégal th. doct. État Sciences, univers. Pierre-et-Marie-Curie, Paris-VI, 248p.
- Lhomme F (1979) Biologie et dynamique de Penaeus duorarum notialis Perez farfante, 1967 au Senegal: III - reproduction. Centre de Recherches Océanographiques de Dakar- Institut Sénégalais de Recherches Agricoles. Document Scientifique N° 69.
- Lhomme F, Garcia S (1984) Biologie et exploitation de la crevette penaeidae Penaeus notialis Pérez Farfante, 1967 au Sénégal. In: A. Gulland and B. J. Rothschild (éds.). 111-141.
- Lhomme F, Vendeville P (1993) La crevette rose Penaeus notialis (Pérez Farfante, 1967) en Côte d’Ivoire. In: Le Lœuf, É. Marchal, J. B. Amon Kothias (éds.), Environnement et ressources aquatiques de Côtes d’Ivoire. I- Le milieu marin. Paris, Orstom, 489-520.
- Thiaw M (2007) La crevette blanche du Sénégal: monographie et méthodes d’étude de la dynamique des populations d’une espèce à courte durée de Agro campus Rennes Ph-D Halieutique.
- Powell C B (1977) A revision of the African freshwater shrimp genus Desmocaris Sollaud, with ecological notes and description of a new species (Crustacea Decapoda Palaemonidae). Revue de Zoologie Africaine, 91:649-674.
- Powell C B (1979) Three alpheid shrimps of a new genus from West African fresh and brackish waters: taxonomy and ecologial zonation (Crustacea Decapoda Natantia). Revue de Zoologie Africaine. 93:116-150.
- Villé JP (1972) Cycle ovarien saisonnier chez Macrobrachium vollenhovenii (Herklots, 1857), Décapode, Palaemonidae, Côte d’Ivoire. Annales de l’Université d’Abidjan, série. E, Ecologie, 5:561-576.
- Champagne R, Andreas K, Anderson J (2007) Généralités sur la biologie et la maintenance des crevettes en eau douce, pour les principales familles et espèces présentes dans notre
- Sintondji SW, Adjahouinou DC, Djihinto GA, Fiogbe ED (2020) Embryology of African giant freshwater shrimp Macrobrachium vollenhovenii. Biologia, 75:93-101. https://doi.org/2478/s11756-019-00280-5
- Cumberlidge N (2006) Description des espèces de crustacés collectées dans le nord-ouest de la Guinée. Rapid Assessment Program (RAP) Conservation International, Washington Bulletin of Biological Assessment / Bulletin RAP d’Évaluation Rapide, 41, Annexe 3, 168-175.
- Garcia S, Le Reste L (1981) Cycles vitaux, dynamique, exploitation et aménagement des stocks de crevettes FAO Document Technique sur les pêches No 203.
- Petit H (2004) La métamorphose chez les Crustacés, un événement Bio future N°249:50-52.
- Mama D (2010) Méthodologie et résultats du diagnostic de l’eutrophisation du lac Nokoué (Benin). Mémoire de thèse de l’Université de Groupement de Recherche Eau Sol Environnement - EA 4330, N° -200.
- Vodougnon H, Lederoun D, Amoussou G, Adjibogoun D, Lalèyè P (2018) Ecologic stress in fish population of Lake Nokoué and Porto-Novo Lagoon in International Journal of Fisheries and Aquatic Studies. 6:292-300.
- N’Zi, GK, Gooré BG, Kouamélan EP, Koné T, N’Douba V, et al. (2008) Influence des facteurs environnementaux sur la répartition spatiale des crevettes dans un petit bassin oust africain - rivière Boubo - Côte d’Ivoire. Tropicultura. 26, 1:17-23. https://doi.org/10.1051/cagri/2021005
- Okpeitcha OV, Chaigneau A, Morel Y, Stieglitz T, Pomalegni Y, et al. (2022) Seasonal and interannual variability of salinity in a large West-African lagoon (Nokoué Lagoon, Benin). Estuarine, Coastal and Shelf Science, Volume 264, 107689, ISSN 0272-7714. https://doi.org/10.1016/j.ecss.2021.107689
- Merbah S (2002) Indices de croissance et d’exploitation de deux espèces de crevettes profondes: Aristeus antennatus (RISSO, 1816) et Parapenaeus longirostris Lucas, 1846 des côtes algériennes (région centre). Mémoire d’ingénieur d’état en océanographie, S.T.H.B:136.
- Slimani Set Hamdi H (2004) Etat des stocks des principales ressources démersales en Méditerranée Groupe de travail du Sous-comité d’évaluation des stocks (SCES) sur les espèces démersales. Malaga (Espagne), 6-7.
- Thiam N, Diadhiou HD, Fall M, Diop M, Thiam D, Barry S (2009) Bioécologie et évaluation des stocks de la crevette gamba (Parapenaeus longirostris) au Sénégal. Rapport commission technique "Eco-biologie et évaluation des stocks démersaux profonds. MEMTMP/DPMCRODT /AFD/ AECID,
- Bekadja IB, Mouffok S, Kherraz A (2009) Boutiba Z Etude préliminaire sur la biologie et la dynamique des populations de la Crevette profonde Parapenaeus longirostris (Lucas, 1846) de la façade Maritime o European Journal of Scientific Research.Vol.36 No.1, 134-144.
- Mouffok S, Kherraz A, Bouras D, Bennoui A, Boutiba Z (2008) Premières observations biologiques de la crevette profonde Aristeus antennatus (Decapoda: Aristeidae) exploitée le long du littoral occidental algérien (Méditérannée du sud-ouest). European journal of scientific research, 19 No.4 (fevrier 2008).817-827, 67.
- Arrobas I, Ribeiro-Cascalho A (1987) On the biology and fishery of Aristeus antennatus (RISSO, 1816) in the south portugueus Investigación Pesquera, 51 (Supl1): 233-243.
- Burukovsky RN (1998) On the distribution of shrimp in West African Russian Journal of Zoology, Vol. 2,No.3, pp 400-408.Translated from Zoologicheskii Zhurnal, vol.77, No. 7, 1998, 778-787.
- Crosnier A, Fontana A, Le Guen JC, Wise JP (1970) Ponte et croissance de la crevette pénéide Parapenaeus longirostris (Lucas) dans la région de Pointe-Noire (République du Congo). Cahiers Office de la Recherche Scientifique et Technique Outre-Mer, VIII, n° 4, 89-102.
- Miller GC (1971) Commercial fishery and Biology of the freshwater shrimp Macrobrachium in the lower Paul River. Liberia, 1952 - 53. US Dept. of Commerce special Sci. Report. N0. 626.
- Villé J P (1971b) Biologie de la reproduction des Macrobrachium de Côte d’Ivoire. III-Description des premiers stades larvaires de Macrobrachium vollenhovenii. Annales de l’Université d’Abidjan, série. E, Ecologie, 4:325-341.
- Holthuis L B (1949b) On some Species of Macrobrachium (Crustacea Decapoda) from West Africa. Eos, Madrid 25 (3-4):175-185.
- Müller Y, Ammar D, Nazari E (2004) Embryonic development of four species of palaemonid prawns (Crustacea, Decapoda): pre-nauplias, naupliar and post-nauplias Rev Bras Zool, 21:27-32.
- De Man JG (1925) Contribution à l’étude des décapodes macroures marins et fluviatiles du Bassin du Annales du Musée du Congo Belge, Zoologie, Série III,Arthropodes Section III—Crustacés 1:1-54, tables A-H.
- Pereira & García DJV (1995) Larval development of Macrobrachium reyesi Pereira (Deacapoda: Palaemonidae), with a discussion on the origin of abbreviated development in palaemonids. Journal of Crustacedan Biology, 15:117-133.
- Wood LE, Daniels SR and De Grave S (2018) Caridina Susuroflabra Richard & Clark, 2009 is a junior synonym of the Widespread Caridina Africana Kingsley, 1882 (Decapoda, Atyidae). Crustaceana 91:243-249. https://doi.org/10.1163/15685403-00003756