Approach Considerations
The purpose of the Nissen fundoplication is to restore competency to the lower esophageal sphincter (LES) while preserving normal swallowing and belching. All procedures are performed with the patient under general endotracheal anesthesia.
Nissen fundoplication can be performed with either an open or a laparoscopic technique. Currently, laparoscopic Nissen fundoplication is considered the criterion standard. [24, 25, 26] Transoral incisionless fundoplication is another alternative being studied. [27]
The following discussion addresses open Nissen fundoplication. This procedure may be approached either transabdominally or transthoracically, as described below.
Transabdominal Approach
The patient is positioned as previously described (see Patient Preparation). An upper midline laparotomy is performed in most cases. Bilateral subcostal incisions may be appropriate in patients who are obese with a wide costal angle. Use of a sternal retractor or various abdominal-wall retractors of choice is necessary. The liver should be retracted anteriorly and to the right with a liver retractor. If the liver is exceptionally large, the left triangular ligament can be divided for further mobilization of the liver.
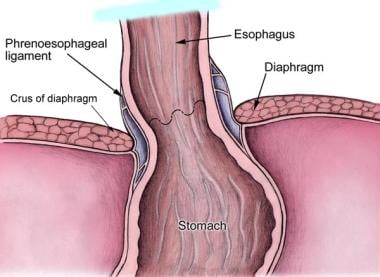
The upper stomach and esophageal diaphragmatic hiatus can now be visualized (see the image below), and the hiatal dissection can begin.
The stomach is retracted caudally. The lesser omentum (also known as the gastrohepatic ligament) is opened above and below the hepatic branch of the anterior vagus nerve, which should be preserved. The incision can be continued superiorly over the anterior surface of the esophagus and down the left crus of the esophageal hiatus. The caudate lobe of the liver and the right hiatal pillar are now visualized. Care should be taken to avoid a possible accessory left gastric artery running with the hepatic branch of the anterior vagus nerve.
The phrenoesophageal ligament is the reflection of the subdiaphragmatic fascia onto the transversalis fascia of the anterior abdominal wall. This ligament is divided.
Freeing the intrathoracic esophagus up to 6 cm may be necessary to gain appropriate intra-abdominal esophageal length. All branches of the vagus nerves should be preserved. The anterior branches have numerous anatomic variations and are included in the fundoplication.
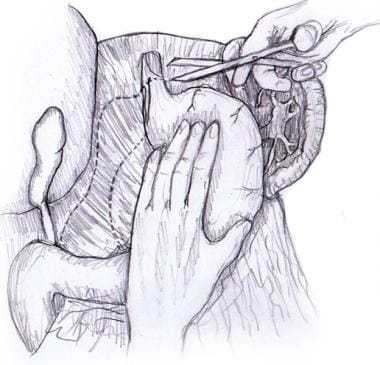
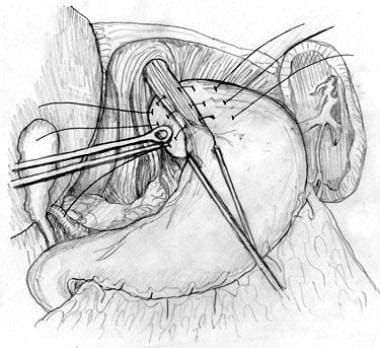
Blunt finger dissection can be used to free the distal esophagus from its posterior attachments. Once the esophagus has been freed circumferentially, it can be encircled with a nylon tape, Penrose drain, or instrument. The esophagus can then be retracted anteriorly to expose the posterior hiatus (see the image below).
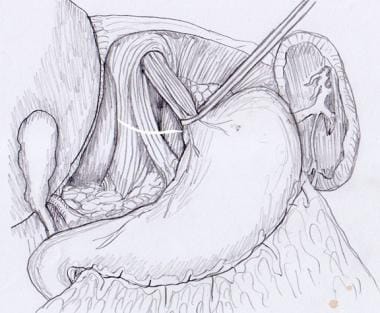 Hiatal dissection. Esophagus is encircled and retracted anteriorly, exposing posterior hiatus for dissection.
Hiatal dissection. Esophagus is encircled and retracted anteriorly, exposing posterior hiatus for dissection.
The hiatus is dissected meticulously to delineate the diaphragmatic crus. The distal 6 cm of the posterior esophagus is fully mobilized. Care should be taken to preserve the inferior phrenic artery; in rare cases, this vessel can be damaged during mobilization of the anterior surface of the fundus. In approximately 5% of patients, the left inferior phrenic artery arises from the left gastric artery and runs along the edge of the right hiatal pillar. In this case, it must be ligated to facilitate hiatal mobilization.
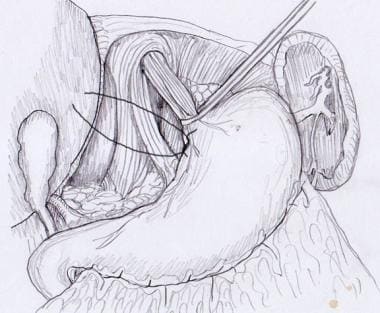
Some surgeons elect to repair the hiatus as needed, whereas others repair it as a matter of routine. Sutures should be placed from posterior to anterior and should narrow the hiatus to approximately 2.5 cm in diameter. (See the images below.) In patients without a hernia or with only a small hernia, one or two interrupted 0-0 nonabsorbable sutures will suffice. More sutures may be needed for larger hernias. Some surgeons may place additional sutures anteriorly or use mesh for repair of large hiatal hernias.
After hiatal repair, the surgeon should be able to freely insert a fingertip adjacent to the esophagus. Patterson et al recommended the use of a 56-French bougie across the esophagogastric junction (EGJ) during the hiatal repair and fundoplication to decrease the risk of postoperative dysphagia. [28] Other authors have reported equivalent outcomes without the use of bougies. [29]
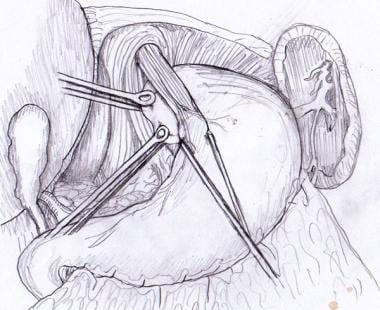
The Penrose drain, nylon tape, or instrument used to retract the esophagus may now be removed. Atraumatic Babcock forceps are used to grasp the fundus of the stomach and bring it behind the esophagus (see the image below).
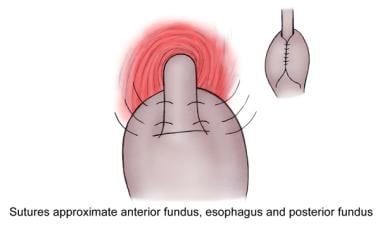
In the traditional Nissen fundoplication (see the image below), the posterior and anterior walls of the stomach are united anteriorly around the gastric fundus to provide a complete 360º 4-to 5-cm wrap around the lower esophagus containing a large intraesophageal bougie. One or two stitches should include the wall of the esophagus to prevent slippage of the cardia. It should be noted that the short gastric vessels are not divided in the traditional Nissen fundoplication.
Once the fundoplication is complete, the abdomen should be inspected for adequate hemostasis. All instruments should be removed. The fascia should be closed with a nonabsorbable suture of the surgeon's preference. The skin is closed with surgical staples.
Technical modifications
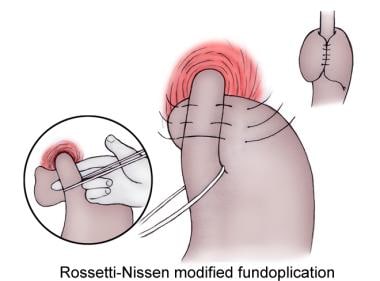
Because of side effects associated with the original Nissen fundoplication, several modifications have arisen. The Rossetti modification uses the anterior wall of the fundus alone to construct a 360º wrap around the distal esophagus. In the initial Nissen-Rossetti modification, dividing the short gastric vessels was not recommended. However, if a tension-free wrap cannot be obtained, the short gastric vessels can be divided. The complete fundoplication should be 2-3 cm in length. (See the images below).
DeMeester and Donohue described a floppy Nissen technique in which the short gastric vessels are divided. [30, 31]
DeMeester et al described three intraoperative measures for decreasing the risk of postoperative complications, as follows [31] :
-
A larger bougie (60 French) decreased the incidence of early postoperative dysphagia
-
Shortening the fundoplication from 4 cm to 1 cm reduced the incidence of persistent dysphagia
-
Mobilization of the gastric fundus allowed improved distal esophageal sphincter relaxation during swallowing
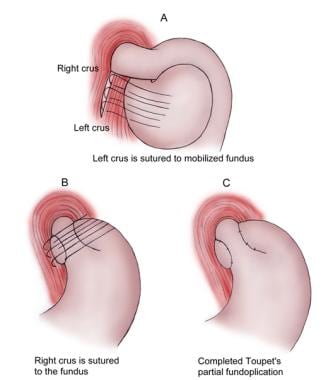
Several other alternative fundoplications are recognized. The Belsey Mark IV partial fundoplication is a 270º anterior transthoracic fundoplication. Dor described a 180-200º anterior fundoplication. Toupet described a posterior 270º fundoplication ideal for patients with motor abnormalities (see the image below). A Thal partial fundoplication is described as a 90º anterior wrap. A Watson partial fundoplication is described as a 120º anterolateral wrap.
Other modifications include narrowing of the esophageal hiatus posterior to the esophagus, repair of associated hiatal hernias using mesh, anchoring of the fundoplication to the preaortic fascia, and the addition of a highly selective vagotomy.
Intraoperative complications
Iatrogenic splenic injury may occur during transabdominal fundoplication. Perforation of a severely inflamed esophagus is also a risk. Care should be taken during mobilization of the esophagus to prevent perforation.
Transthoracic or Thoracoabdominal Approach
Because of concerns about morbidity, a thoracic approach is rarely used. The main advantages of the transthoracic approach are that it provides direct vision of the lower and middle esophagus and easier mobilization of the middle esophagus. This approach may be appropriate for the following patients:
-
Those who have previously undergone previous hiatal hernia surgery
-
Those with a need for concomitant esophageal myotomy for achalasia or diffuse spasm
-
Those who have a short esophagus
-
Those who have a nonreducible sliding hiatal hernia
-
Those who have concomitant pulmonary pathology that can be inspected simultaneously
A left posterior lateral thoracotomy between the sixth and eighth intercostal spaces is used for the transthoracic approach. In the thoracoabdominal approach, the patient is positioned with the left side raised so that a midline laparotomy can be supplemented by a left anterolateral thoracotomy.
Selective deflation of the left lung with a double-lumen endotracheal tube may assist in visualization. Mobilization of the esophagus begins with incision of the mediastinal pleura and encircling of the esophagus with a Penrose drain. Mobilization of the esophagus to the aortic arch is necessary to allow a tension-free repair and to achieve adequate esophageal length in the abdomen. If the length is not adequate, then an esophageal-shortening procedure (eg, a Collis gastroplasty) is performed. [32]
The left superior and inferior bronchial arteries comes directly off the descending thoracic aorta to the lower esophagus and are ligated. The cardia of the stomach is dissected free from the diaphragm. Usually, this can be accomplished by dissecting transhiatally. If necessary, however, the diaphragm can be circumferentially incised for additional visualization. The abdomen is entered through the phrenoesophageal membrane along the anterior left crus. Care should be taken to avoid the gastric vessels.
The left hand is inserted through the diaphragmatic hiatus around the esophagus. A combination of blunt and sharp dissection can be used to free the esophagus distally. Next, a portion of the body of the stomach is retracted up through the hiatus into the chest with Babcock clamps. Often, this requires ligation of four to six short gastric vessels. Once the fundus is elevated, the posterior gastric artery is visualized and ligated. The vascular fat pad localized on the anterior lateral surface of the cardioesophageal junction is excised.
Depending on the surgeon's preference, crural sutures may be placed as described above for the transabdominal approach. These sutures may be placed either before or after the fundoplication. If these crural sutures are placed before creation of the fundoplication, they should be tied after creation of the fundoplication has been completed.
The fundoplication is created next, in much the same fashion as with the transabdominal approach. The fundoplication is then placed into the abdomen. The hiatus is inspected to confirm that the repair remains completely intra-abdominal. If the fundoplication does not stay intra-abdominal, excess tension on the repair is likely, and revision by further mobilization of the fundus is indicated. If crural sutures were placed, they should be tied once the fundoplication is satisfactory. Otherwise, crural sutures can be placed at this point in the procedure.
-
Rossetti-Nissen modified fundoplication.
-
Nissen fundoplication.
-
Toupet partial fundoplication.
-
Hiatal dissection. Esophagus is encircled and retracted anteriorly, exposing posterior hiatus for dissection.
-
Hiatal repair. Sutures should be placed from posterior to anterior.
-
Hiatal repair.
-
Anterior wall of gastric fundus is passed behind esophagus with atraumatic Babcock forceps.
-
Rossetti-Nissen fundoplication.
-
Anatomy of lower esophageal sphincter.
-
Original Nissen fundoplication, as described by Dr Rudolf Nissen.
-
Severe distal reflux esophagitis, as seen via esophagogastroduodenoscopy. Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.